6 main concerns about changing your eClinical software provider
May 21, 2021
Changing your software provider is a massive burden since there were already many resources invested into picking your current one, implementation, etc. We talk to potential clients nearly 24/7 and 365, and we’ve heard all the concerns and troubles that exist.
So, here is a six-step checklist of the most prevalent concerns before changing a software provider and how we deal with it, making the process smooth and painless.
Let’s see it all in more details.
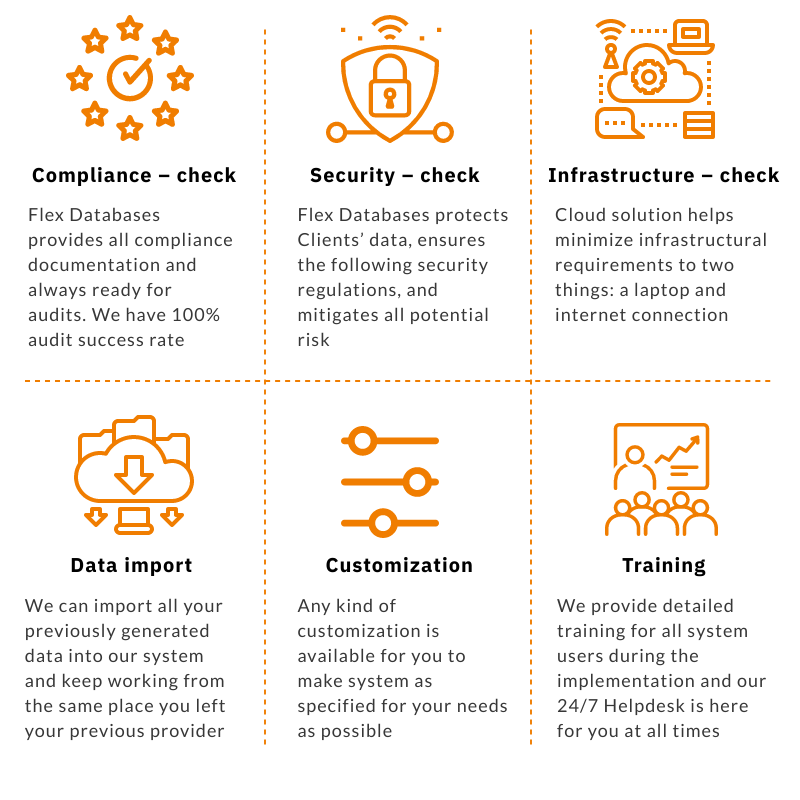
1. We are in the middle of a big project and need to make sure that all data is saved and moved to a new system.
All modules of Flex Databases system have import and integration options – you may choose the way you would like to move your data, and it will be done quickly and securely. Nothing is lost, and you can keep working without any operational breaks. Things kept in paper? Not to worry – we’ll explain how to transition to digital quick and easy.
2. We do like the new provider, but our old system was customized for our needs.
Flex Databases system is, well, flexible. The system is configurable to recreate your processes and not the other way around. Given that you can rename fields, change trackers, workflow steps, templates, etc. if there is something we haven’t thought about, we are ready to either add it to baseline in the next release or customize it for you.
3. Our employees are used to the old system, and new implementation looks like a burden.
While it is a burden, Flex Databases implementation only takes 3-6 weeks. Our team walks you through all the steps, provides all needed documentation, and even literary sits with you through the end-user validation system acceptance part.
We provide detailed training for every module you purchase to welcome your employees into the Flex Databases system. In addition to that, you have our 24/7 in-house Helpdesk ready to answer any questions.
4. Our provider should be compliant with regulations and transparent.
During the vendor selection process, the first question that usually pops to mind is compliance and overall vendor transparency. Is the company compliant with local and global regulations? How can we see it all and make sure everything is correct?
We have a simple answer: Flex Databases provides all the documents you need to ensure our regulatory compliance and 24/7 ready to conduct any type of audit on the shortest possible notice. We have a 100% successful completion rate for all audits undertaken with us and are ready to go the extra mile to ensure that all necessary documentation is presented for customers.
Bonus point – if you have an inspection or an audit, we are always there to support you, answer questions, provide additional documents and explanations.
5. Our data should be secure and protected.
The second thing you usually think about is security. We protect Clients’ data, ensure following security regulations, and mitigate all potential risks, which is essential to building trust and delivering a high level of service. Prior to the contract, we discuss and outline all security questions, such as where your server will be, how we perform backups, our security policies, procedures, etc.
6. We don’t have an IT department in-house.
What infrastructure will we need? Do we have to take additional measures to ensure its proper performance and stability?
With the Flex Databases system, the only thing you need is your laptop and internet access – we provide a cloud solution and take care of infrastructure.
If you want to install your system on your premises – that is also an option! We provide all requirements for infrastructure to ensure that everything runs smoothly.
Got any more concerns, or ready to change your provider?
Contact us through bd@flexdatabases.com or send a demo request via the button on top of the page.



