CTMS 2022 – intelligence insights, not tools for automation
January 14, 2022
Insights on what makes the most impact on the project.
Instant full picture in one interface. Sense of full control.
Clinical Trial Management System (CTMS) is one of the most popular and widely used eClinical software applications. It’s been on the market for decades, and from simple process management, the concept grew in many different directions. What happened to Flex Databases CTMS and where we are going now?

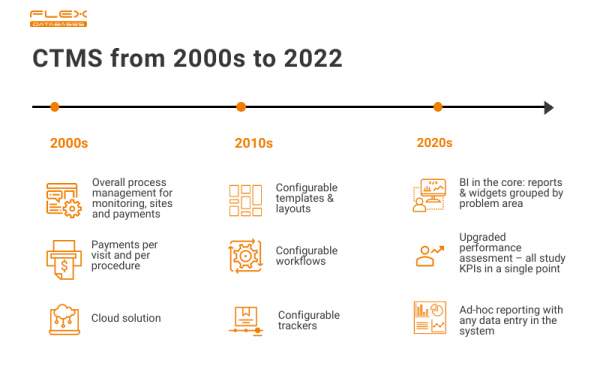
Where it all began
In the very beginning, Flex Databases CTMS was pretty basic to us, but not at all basic for the market. We were among the very first providers, who allowed per visit and per procedure invoices to sites, and it was a huge deal in terms of site payments optimization. We’ve also introduced offline reporting capabilities early, even though the system was cloud-based from the start. It was a great run, we’ve helped many companies, including Novo Nordisk, but the time for innovation has come.
From flexible to fully configurable
As you probably figured from our brand name, Flex Databases CTMS is, well, flexible – we’ve always allowed more in terms of configuration, compared to our competitors. The thought behind that one is simple – you don’t have to align with system architecture, system architecture has to align with you.
At this stage of our system’s development, we decided to go all in. The system now fits your processes like a second skin and provides you with a helicopter view to stay informed 24/7. Everything is configurable. No extra development is needed.
Here’s how it works: configurable tool means the ability to take a process, like Site Visit Report creation, and spin it however you like – set up new trackers, change or add steps, create documents templates, etc. Users driven configurations are free, and you can perform them at any time you want.
Does it sound great? It surely is.
But there’s more.
Upcoming version release: one dashboard for full picture

You don’t have to look for insights anymore – you’ll get them all in one dashboard. The only thing you need is data in the system, and the system will take care of the rest. No need to spend time talking to many people or combing through endless reports – we’ve done it for you.
Here’s a scope of what you’ll get:
- Advanced analytics: risk indicators, predictive analytics, trends analysis
- Upgraded KPI tracking: all study-related KPIs in a single point
- Possibility to add own custom KPIs for any area
- All visuals are grouped by problem area for easier focusing (e.g. start-up, enrollment, deviations, CRAs performance)
- Default pre-configured set of widgets per each area representing industry-standard KPIs
- Risk-based as a philosophy
- Fancy cross-project reports
To put it simply, we are moving forward to data-based CTMS – with 100% transparency of what’s going on in your processes.
Are you looking for a flexible, data-based CTMS? Consider Flex Databases and send us a demo request now.



