User-centric CTMS. C-level, Manager or a CRA? Have we guessed your needs?
October 14, 2021
When we build a product, we base its core on the challenges our customers may face and how to solve them. We step in your shoes to see things from your point of view – what you need.
Here’s the list of challenges that you have, and we solve – and how exactly we solve them:
C-level point of view
I need complete transparency in my processes
- Any data point can make a report – to provide you with an answer to any question
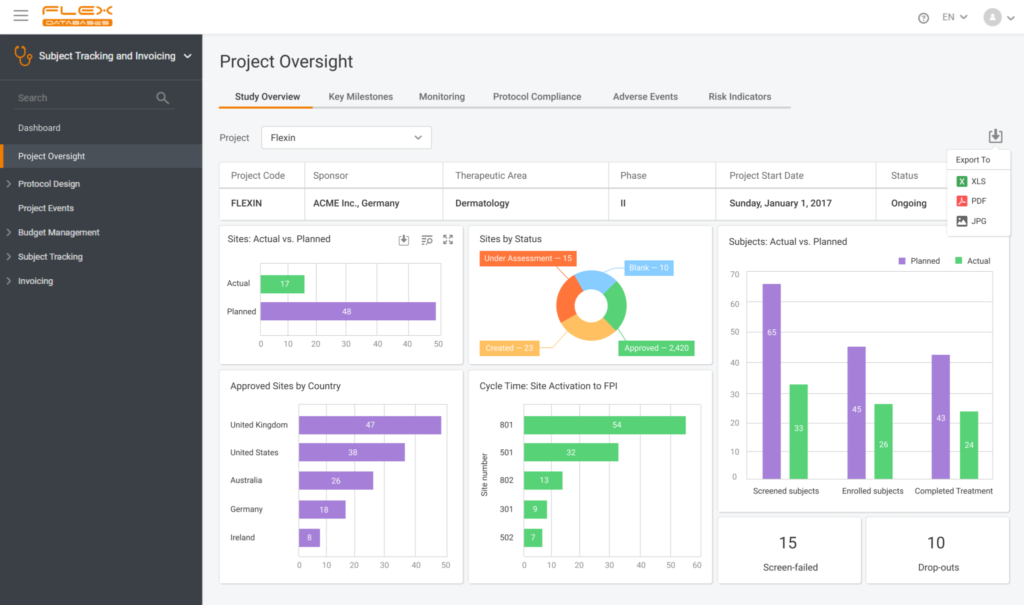
I want to have a helicopter view to see the full picture of what’s going on
- Each module of Flex Databases CTMS has interactive dashboards with the ability to pin any report or process status to it. Widgets, reports, and graphs, all exportable and at your disposal
I want to have the highest possible level of compliance
- Flex Databases CTMS is fully compliant to global and local regulations, provides leveled access control, and ensures complete compliance
I’m aiming for a modern business approach
- We provide advanced technologies in a simple interface – so you can digitalize your clinical trials with a few simple clicks
Manager point of view
I need all documents to be reviewed and approved on time
- Create, review, and approve workflow with electronic signatures is flexible and configurable to replicate your exact processes. Flexible notifications are here to remind users about upcoming deadlines
I need to see the schedule of visits, milestones, enrollment, approaching deadlines, monitoring plan, action items by status and responsible person, deviations, other trackers, all in one place, the performance of CRAs, tasks view, sites per status, where are CRAs with their visits
- You can pin any report or widget from the system to the dashboard and get a complete picture of what’s going on in your study – calendars, milestones, plans, action items, etc
I need to quickly see what is required from me – my tasks and action items
- My Tasks space contains everything that’s to do for you – and customizable notifications won’t let you miss a thing
I want to see KPIs and statistics on CRAs performance
- Flexible smart trackers include action items, issues, deviations, etc. – there is no limit to what you can track and measure
I want transparency of what is going on at my sites
- Track what is going on at sites: enrollment, inclusion curve, in and out of time window visits, screen failures, and so much more in one place
I want reports on performance for my sponsor
- Planned vs. actual reports are everywhere on every data point you can think of
The sponsor wants to review and approve reports
- Review-Approve workflow for Site Visit Reports is inbuilt in the system and fully customizable to fit your SOPs or inner procedures
Reports and documents get lost
- Missing documents report is here to help
Things are out of control, and I need to regain it
- Are things out of control? KPIs are tracked, alerts are set for you to never miss a bit of a quick running study. Monitoring, site information, and site payments are set, measured, organized, and tracked
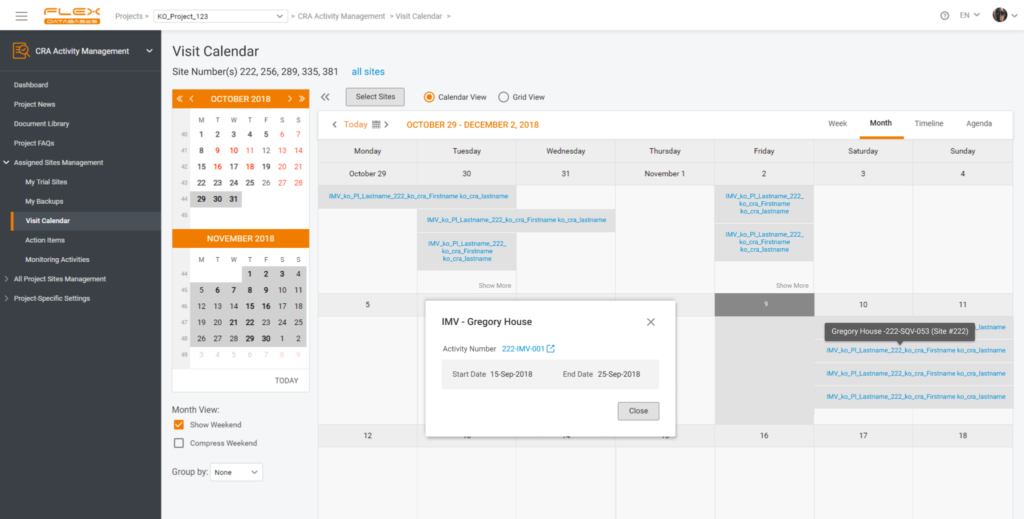
CRA point of view
I need an easy tool to fill in questionnaires
- Flex Databases Site Visit report creation tool is user-friendly and follows the flow set within your company
Usually, the internet access on sites is poor. I need to work on the go and to have a possibility of offline reporting
- Poor internet connection at the site? Use offline reporting capabilities of the system
I need to see action items and deviations, enrollment, and missing documents for the site before a visit. Ideally, it is prefilled in the CL, FUL, reports. Ideally, everything is written by the system, I only check the information, and it recalculates automatically based on EDC data.
- All action items are here on your dashboard for you – and you can edit it however you’d like, pin or unpin something you use frequently
I want to generate documents with one button and simply send them for review.
- Create Confirmation Letter, Follow-up Letter in one click with all related trackers added automatically. All templates and documents are version-controlled and automated
I want to receive alerts for timelines, visits, review of the documents’ status
- Hard to keep track of all visits? Use visits calendar and your tasks tab. Flexible notifications will alert you of anything that requires your attention
I need to see all site-related data in one place.
- Sites statuses, addresses, documents, contracts, qualification, vendors, submissions, site staff, timelines, and so much more at hand
I am in a rush, and I need a system that can support me and not slow me down
- Flex Databases CTMS is built for users – to make routine tasks easier, so you can distribute your workload more efficiently
Flex Databases CTMS provides:
- Work done in one place, measured, organized, reported
- Inspection readiness and compliance while easy to use
- Advanced technology in a simple interface
Do you have any challenges that we didn’t mention here? There’s much more! Check out Flex Databases CTMS product page for additional information.
If you’d like to see the system in action, here are the short demo videos:
Are you looking for something similar? Book a demo through our website or send us a request to bd@flexdatabases.com to learn more!



