




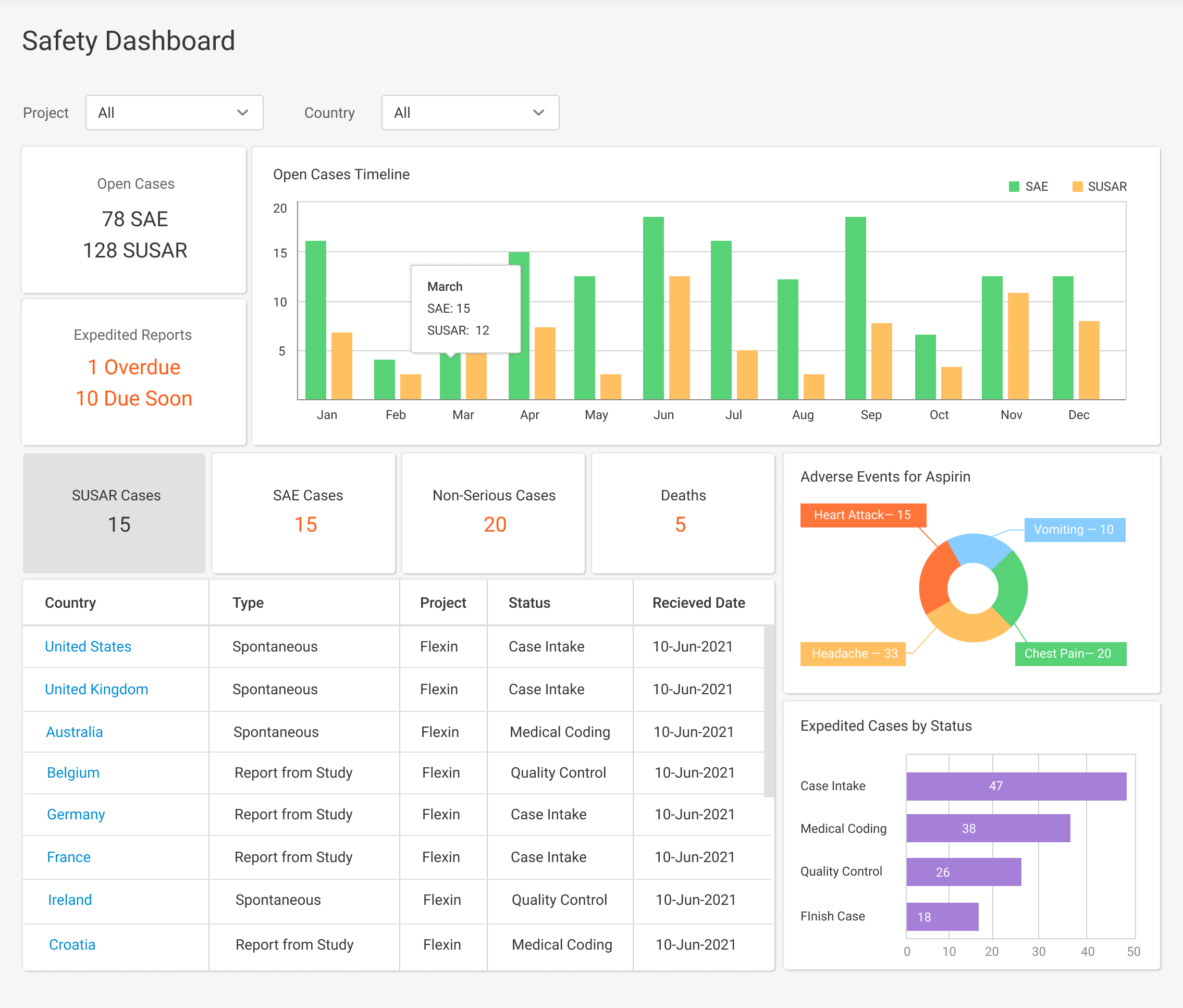
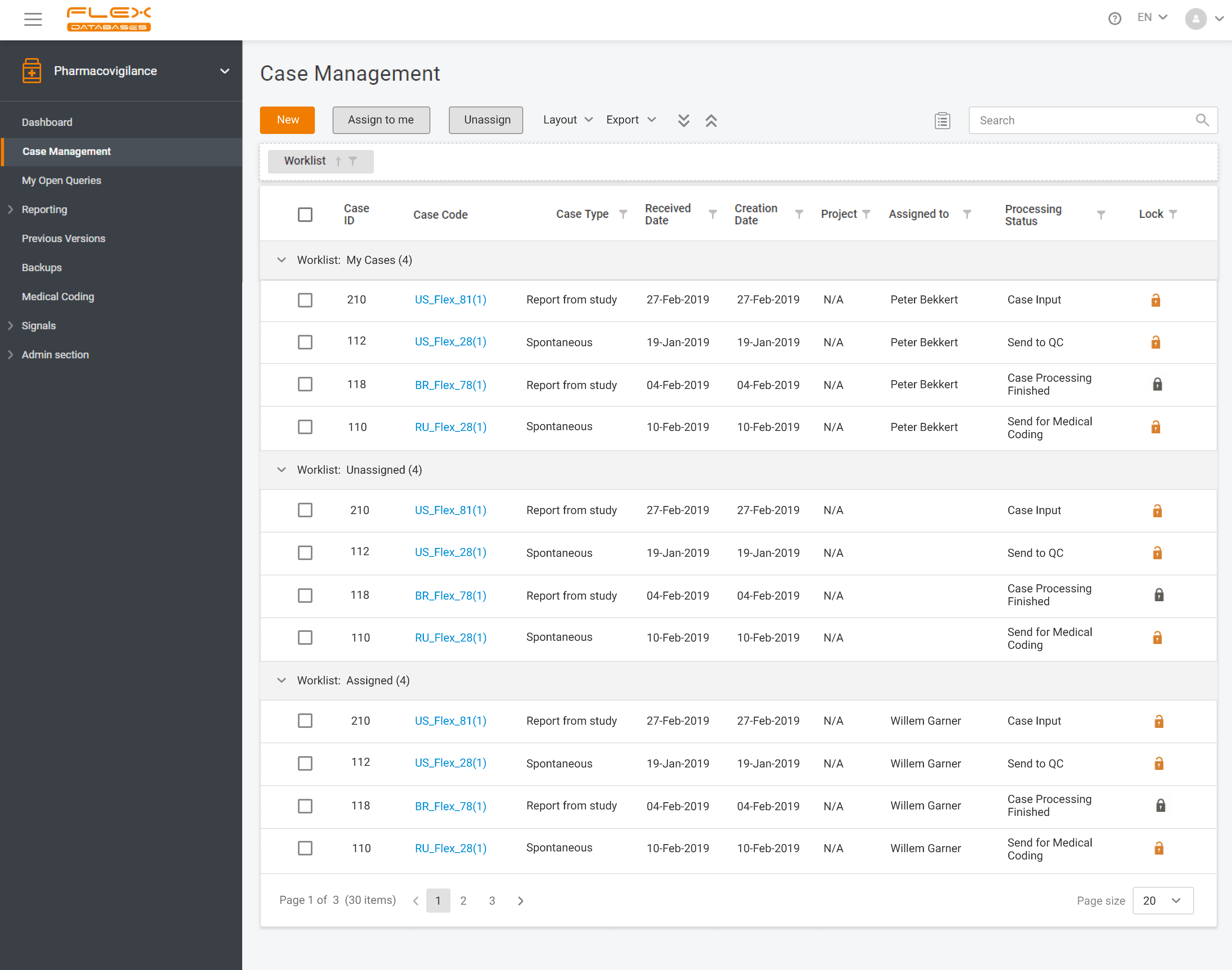
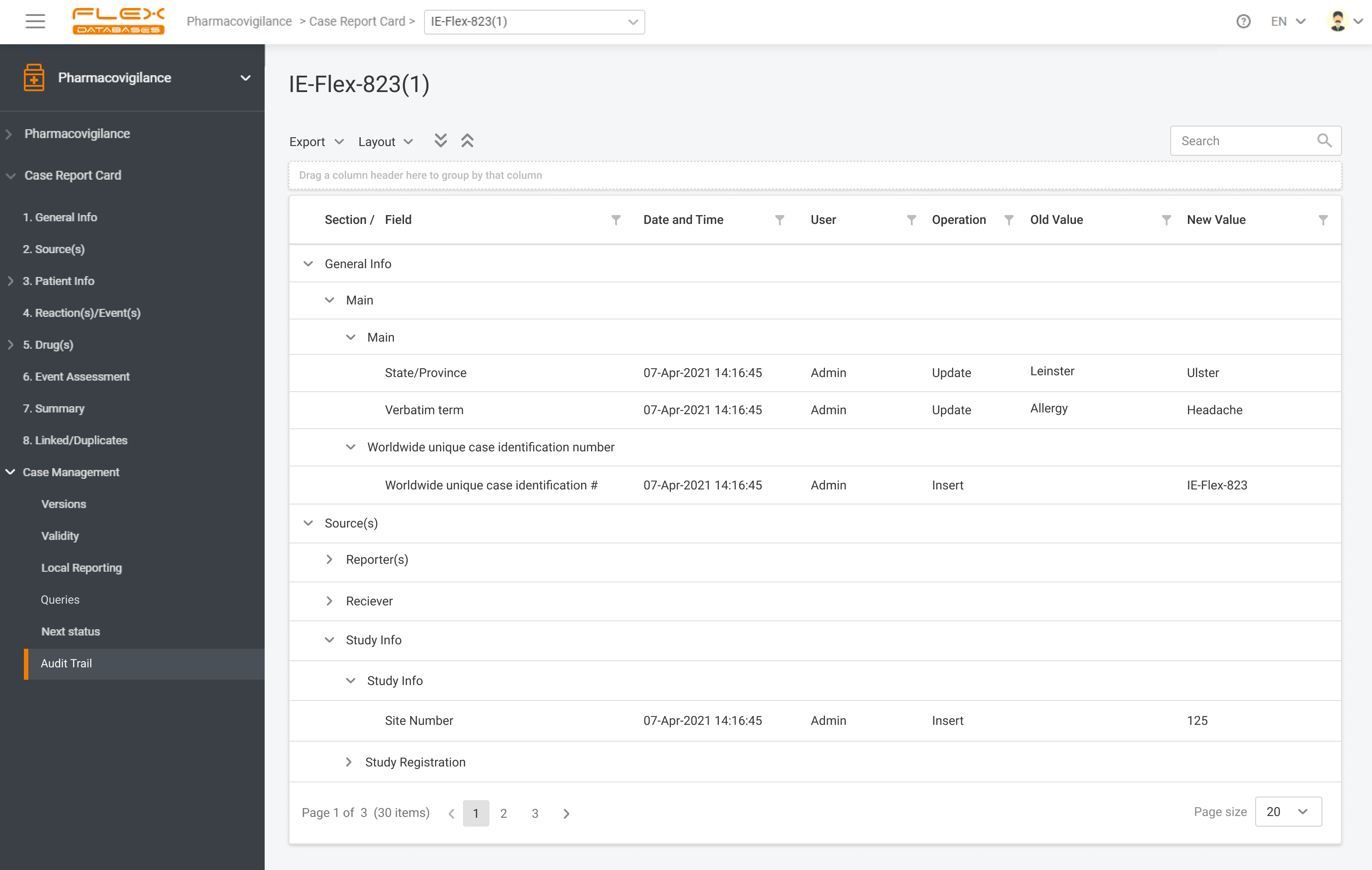
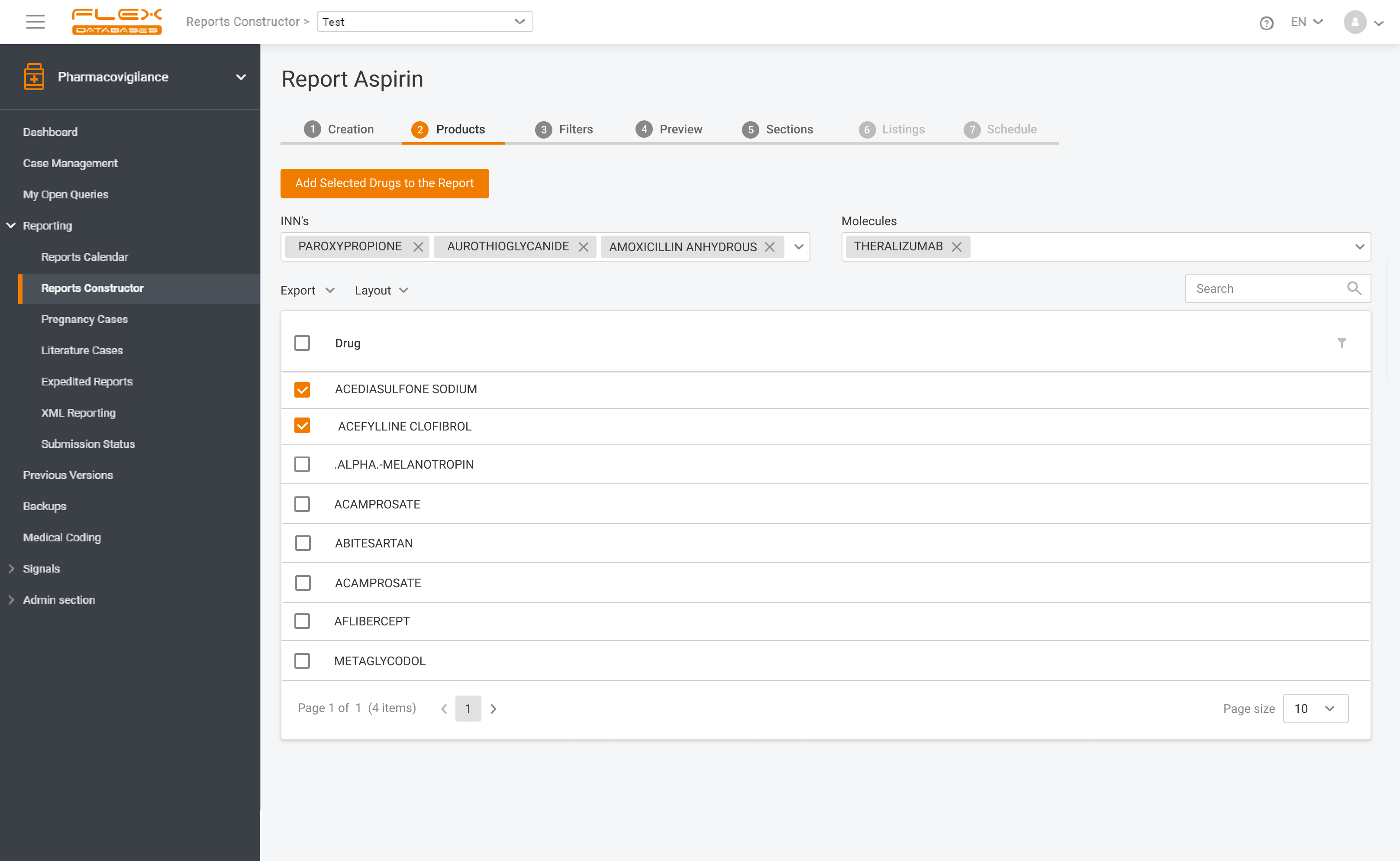
Flex Databases Pharmacovigilance is a comprehensive drug safety database that ensures robust and compliant pharmacovigilance activities both within clinical trials and post-approval stage. End-to-end process of collecting, triage, evaluation and submission of safety data in a single point.





Drop us a line and we be happy to answer all your questions
Clients
Daily users
Documents
Clinical trials
Countries
Cross-site budget setup can turn into hours of manual per-subject cost entry, slowing teams down and creating error risks. With Flex Databases’ Subject Tracking & Invoicing, you can now import Per Subject Costs directly from Excel using the Data Import Wizard. ✔ Speed up site budget setup✔ Reduce manual entry and errors✔ Import across projects […]
In July 2025, the European Commission adopted Commission Implementing Regulation (EU) 2025/1466, amending Regulation (EU) No 520/2012. The changes aim to strengthen pharmacovigilance, reduce unnecessary administrative burden, and align EU requirements with global best practices. Most provisions apply from 12 February 2026, with certain Eudravigilance updates taking effect in August 2025. Why the Changes Were […]
Palobiofarma is a leading clinical-stage biotech company dedicated to developing innovative medicines. Our focus on adenosine signaling drives our commitment to scientific excellence, with six drug candidates currently in different phases of development. Among them, PBF-999 for Prader-Willi Syndrome stands out as a key project. Our mission is to advance patient care by leveraging cutting-edge […]
Pharmacovigilance (PV) is a critical aspect of drug safety, focusing on detecting, assessing, understanding, and preventing adverse drug reactions or other drug-related problems. As global regulatory requirements for drug safety evolve, adopting reliable pharmacovigilance software has become essential for ensuring compliance and improving operational efficiency. Selecting the right PV software can significantly impact your organization’s […]
Get in touch to discuss compliance, implementation, demos, pricing
We are here for all of your questions! Tell us more about yourself and we will organize a tailored live demo to show how you can power up your clinical trials processes with Flex Databases.