Flex Databases Platform: Interface Overview
September 22, 2021
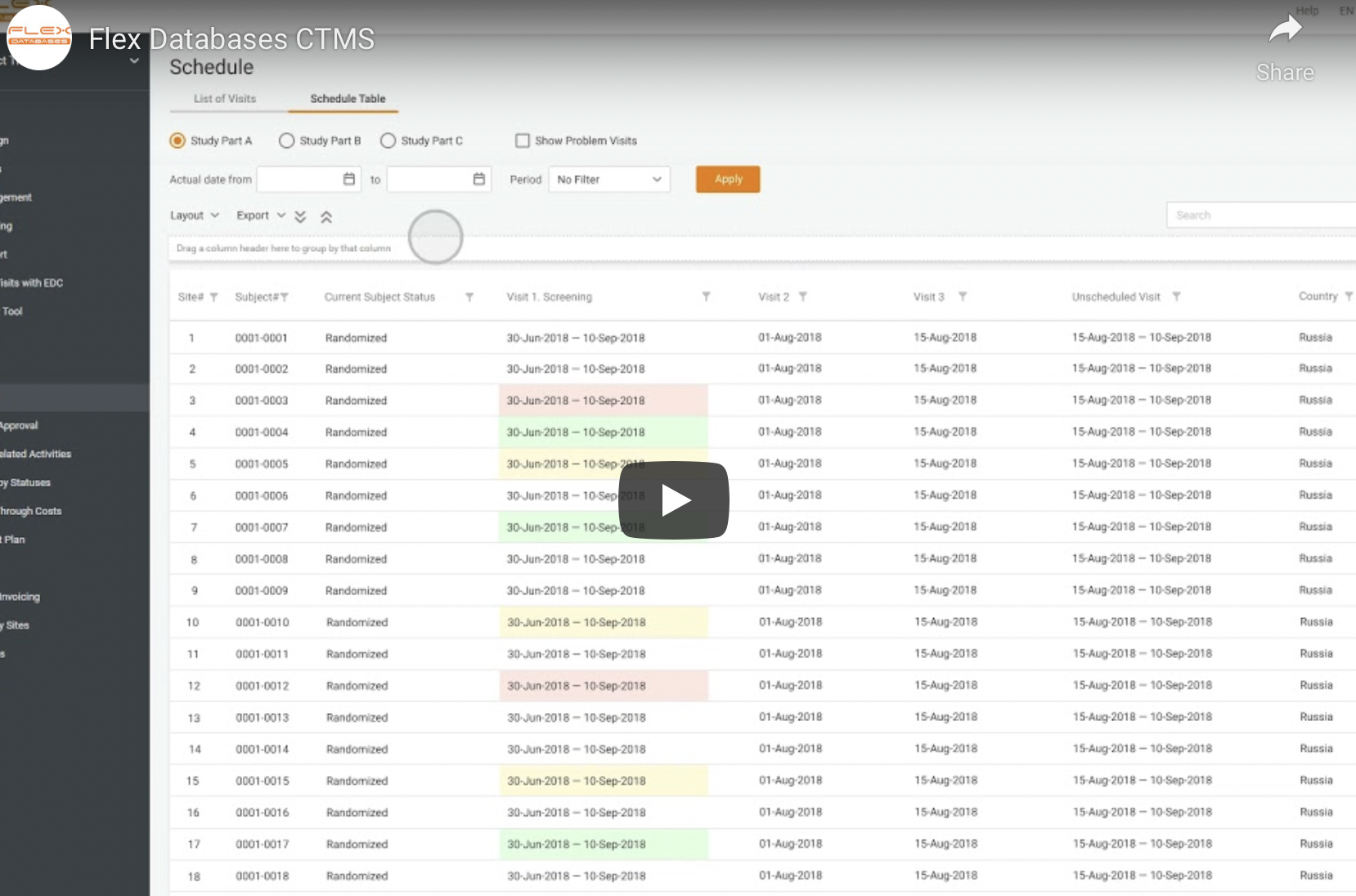
Clinical Trial Management System (CTMS)
Manage all your CRA activities, patients’ data, sites, and invoicing in real time.
- Schedule monitor visits, assess CRA performance, and generate any type of visit reports.
- Set up online invoicing, payment rules, and different site budgets.
- Organize all the information on investigators, sites, vendors, therapeutic areas in one place.
Project Management & Budgeting
Make the complex financial part of your project easy
- Plan and allocate resources and workload for an unlimited number of projects.
- Track and analyze project performance with automated reporting.
- Manage all project-related expenses in one place.
Electronic Trial Master File
Gain complete visibility into all of your trial documentation
- Get instant safe access to your trial documentation from any place in the world.
- Ensure constant audit readiness of your TMF.
- Upload documents in seconds, see missing files, review, and correct everything in real time.
Pharmacovigilance
Ensure robust and compliant pharmacovigilance activities throughout all the trial stages
- Manage all PV processes within one system.
- Communicate with partners and regulatory authorities directly via EDI gateways.
- Make reports in any format, required by regulatory authorities.
Learning Management System
Maintain and improve personnel qualification level
- Organize easy onboarding of new employees with automatically assigned training.
- Update QA documentation & SOPs, notify employees in real time, and set up automated knowledge check-up.
- Get a complete view of your employees’ knowledge level with advanced reporting.



