




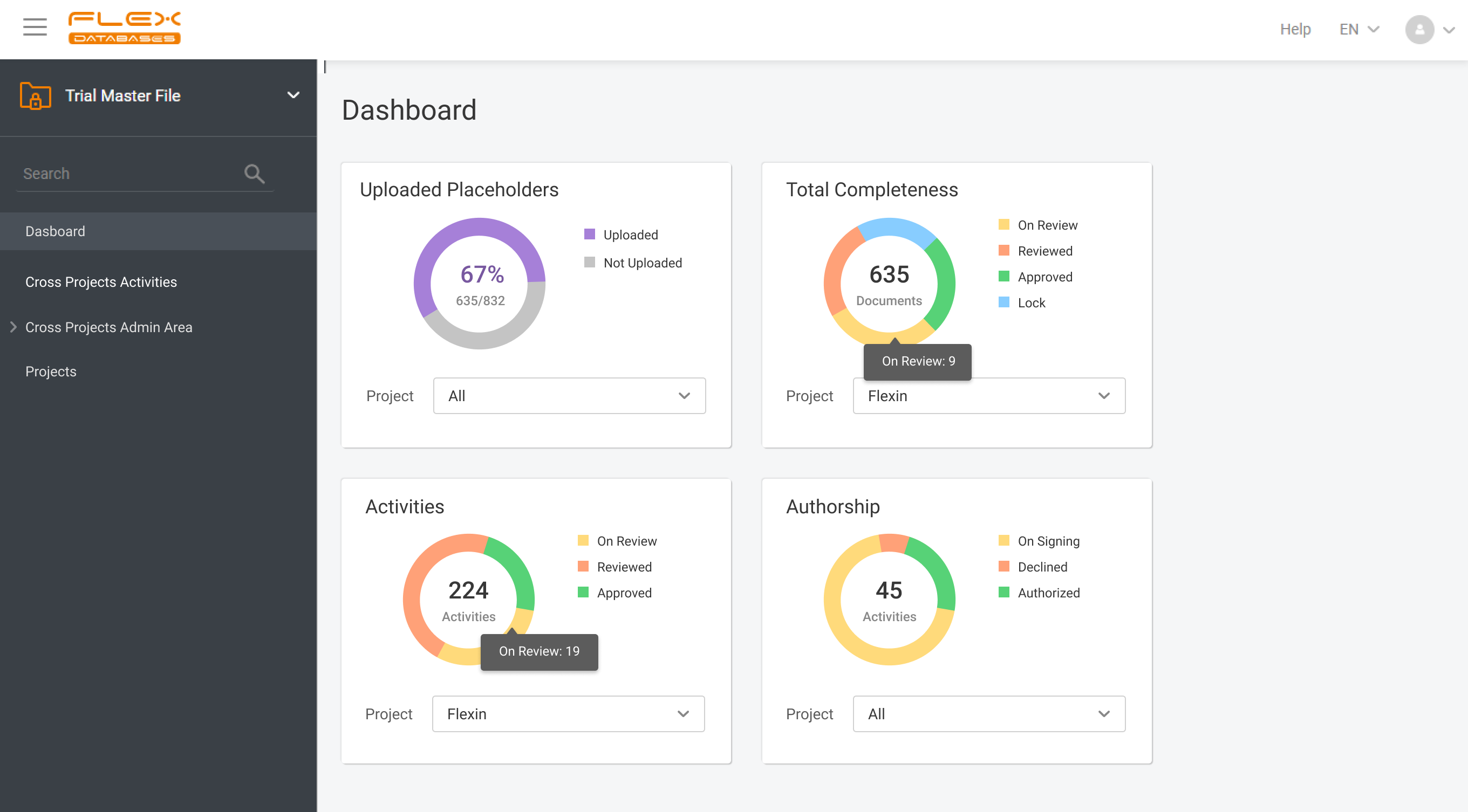
eTMF – complete 24/7 visibility into all your trial documentation





Drop us a line and we be happy to answer all your questions
Clients
Daily users
Documents
Clinical trials
Countries
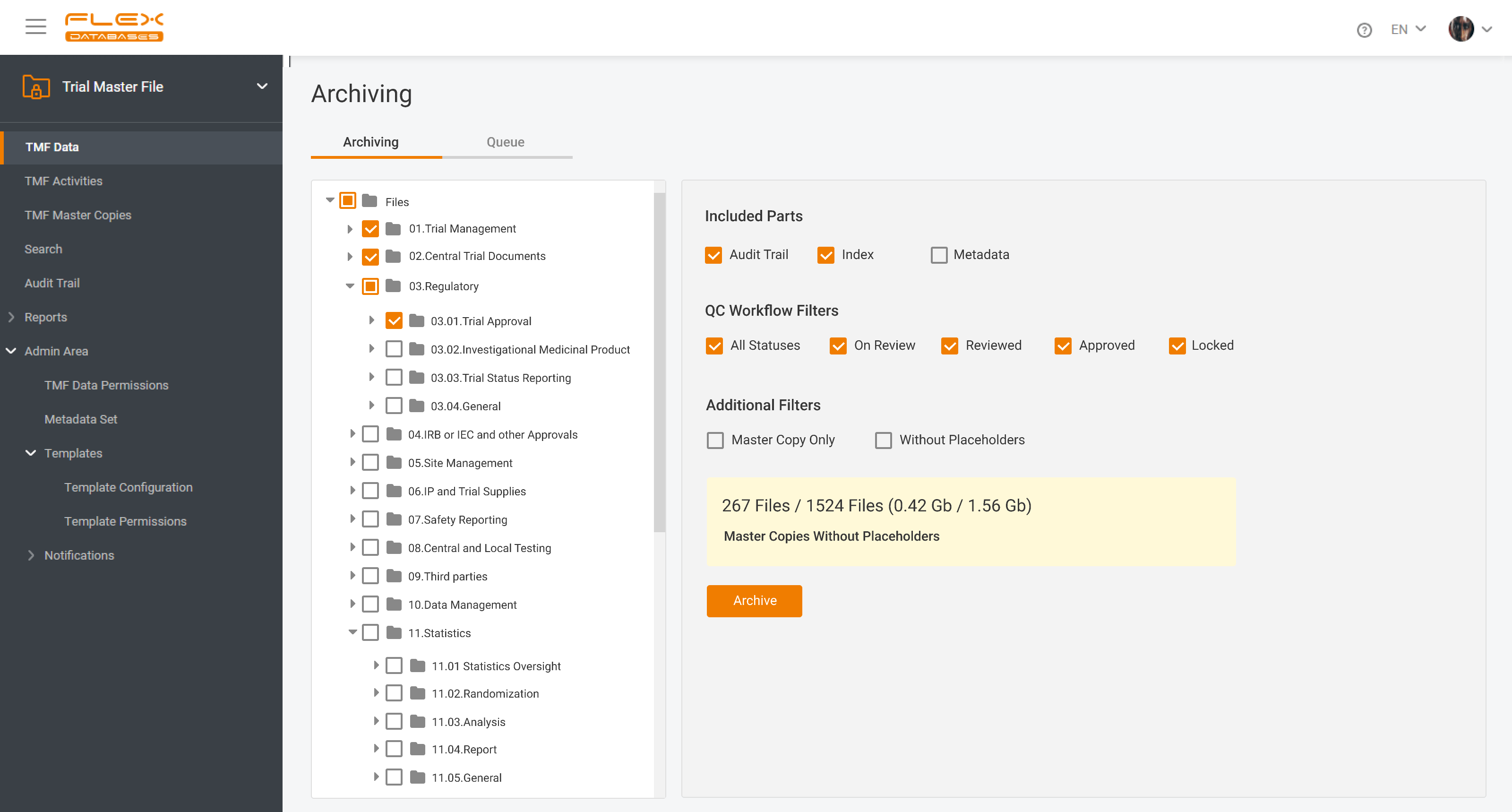
Flex Databases Solves One of the Biggest Document Management Headaches Clinical teams have long struggled with one persistent problem: managing TMF documents across multiple systems, versions, and stakeholders. Switching between platforms, downloading files, losing track of versions, and waiting for colleagues to finish editing slows down operations and increases compliance risks. With this update, Flex […]
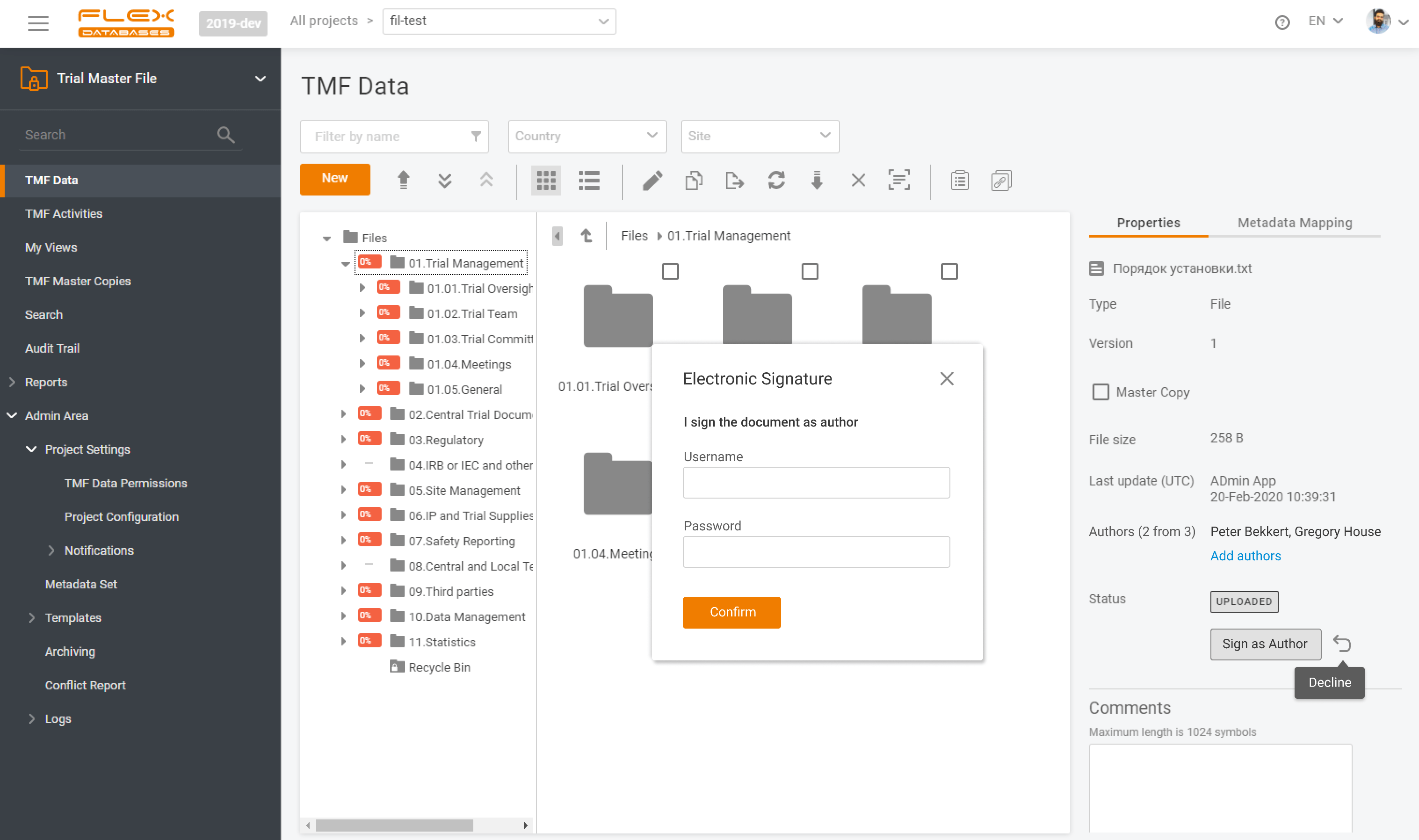
📂 Last-minute TMF quality reviews cause stress, missed documents, and higher audit risks.With Flex Databases, you avoid the scramble – reviews are structured, transparent, and always under your control. With Flex Databases, you get:✅ Control over the process✅ Full transparency and traceability✅ Less stress before audits✅ Clear outcomes in one report 🎥 See how it […]
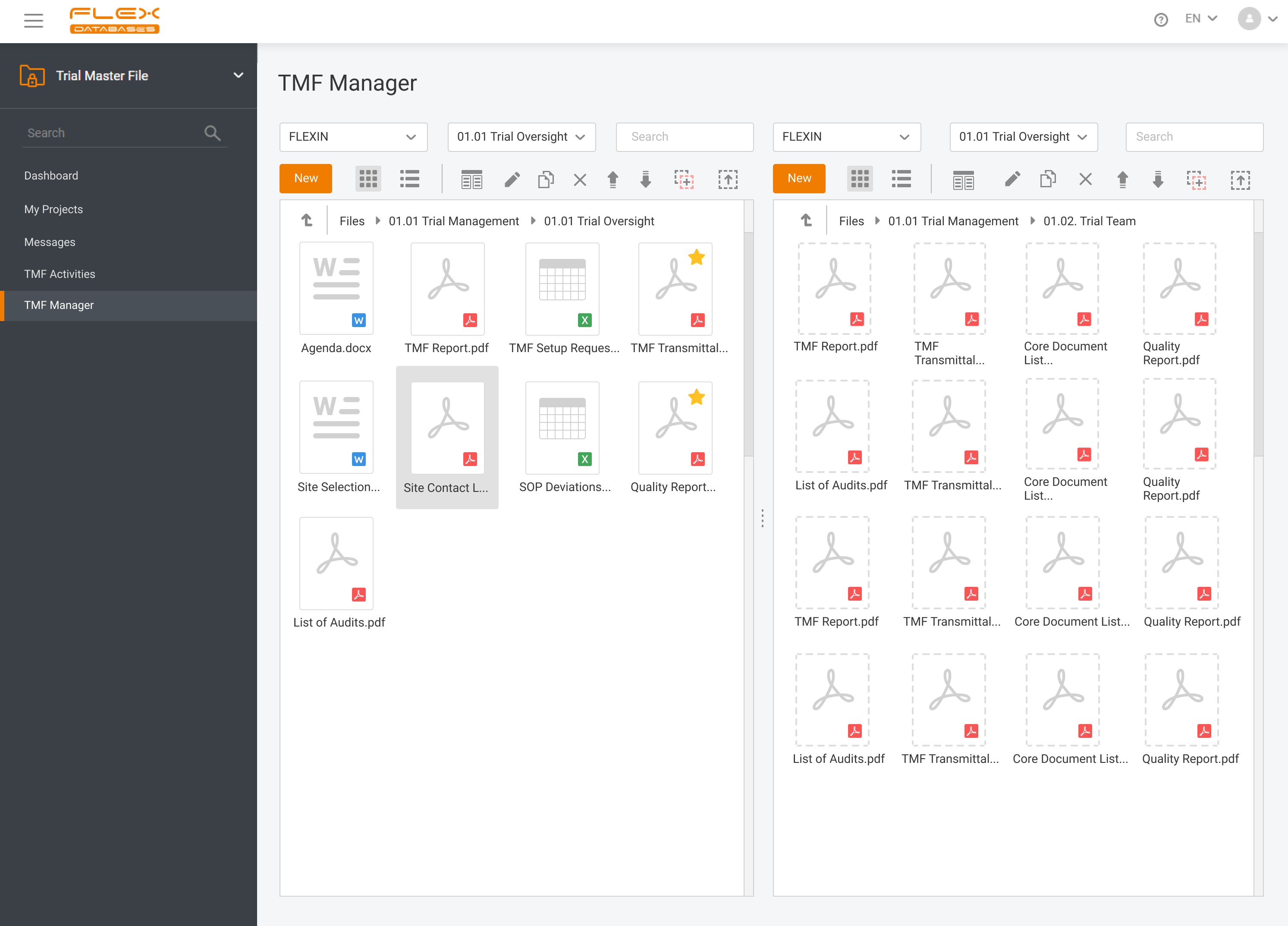
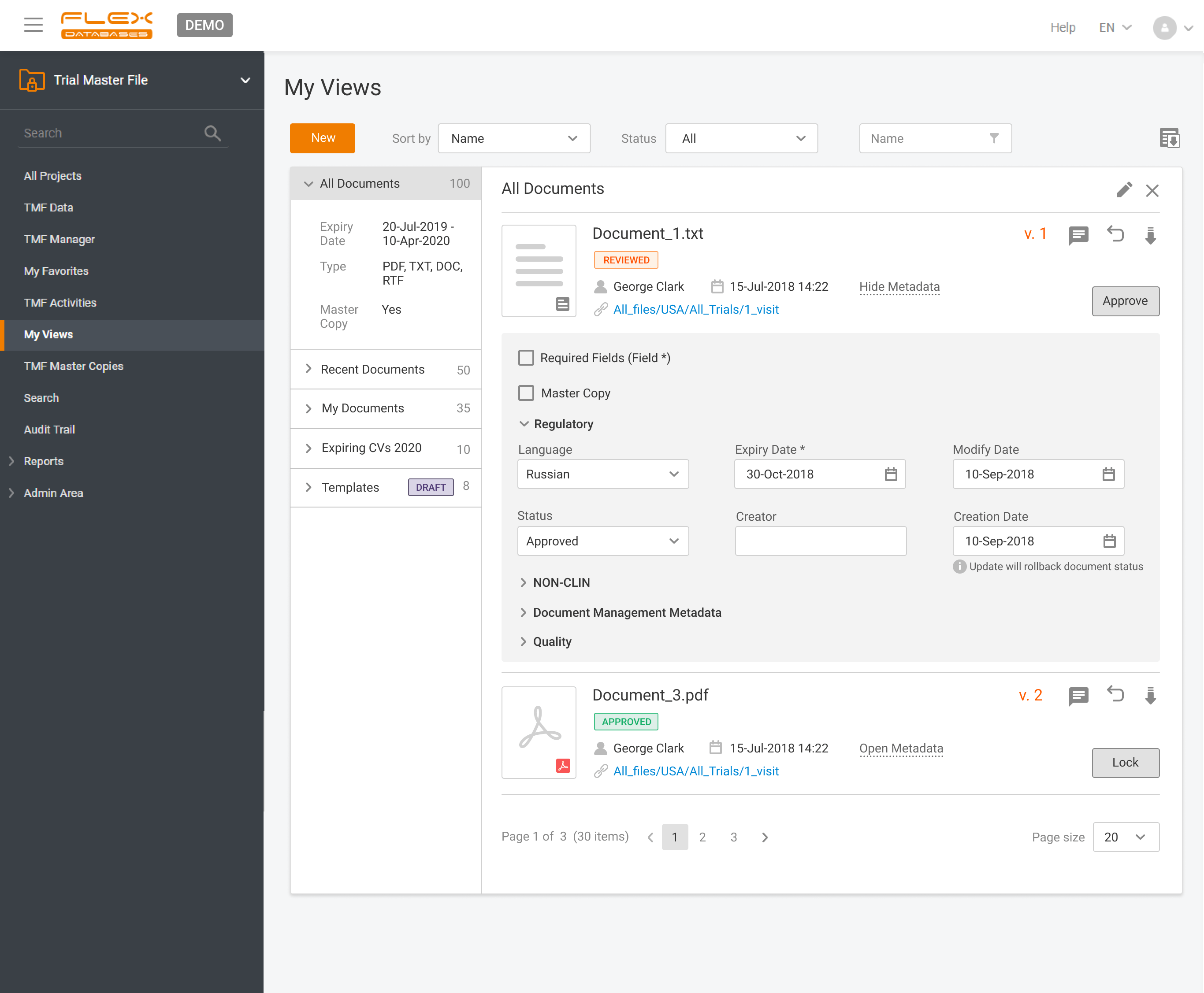
Ever uploaded a document to your Trial Master File only to realize it was already there?You’re not alone! Duplicates waste time, clutter your TMF, and create compliance risks. That’s why we built duplicate detection into Flex Databases eTMF. ✅ Real-time scanning as soon as you upload✅ Checks both file name and document content✅ Skip, replace, […]
Ever uploaded a document to your Trial Master File only to realize it was already there?You’re not alone! Duplicates waste time, clutter your TMF, and create compliance risks. That’s why we built duplicate detection into Flex Databases eTMF. ✅ Real-time scanning as soon as you upload✅ Checks both file name and document content✅ Skip, replace, […]
Get in touch to discuss compliance, implementation, demos, pricing
We are here for all of your questions! Tell us more about yourself and we will organize a tailored live demo to show how you can power up your clinical trials processes with Flex Databases.