How to Choose the Right eTMF Model
May 15, 2025
An electronic Trial Master File plays a central role in maintaining oversight, ensuring regulatory compliance, and supporting successful inspections by health authorities. An efficient eTMF is no longer optional – it’s essential.
Choosing the right eTMF model for your clinical studies directly impacts how effectively your teams can manage documentation, maintain version control, and collaborate across sites and partners. A well-suited model helps streamline workflows, reduce risk, and enhance audit readiness. Flex Databases offers a fully compliant, highly configurable eTMF solution that adapts to a wide range of trial designs and operational models – making it a reliable choice for both sponsors and CROs.
Understanding eTMF Models
Choosing the right eTMF model starts with understanding the main structural approaches commonly used across clinical trials. Each model comes with its own benefits and challenges, depending on your operational setup, outsourcing strategy, and document oversight needs. Below is an overview of the most common eTMF model types and how Flex Databases supports them.
Centralized vs. Decentralized eTMF Models
Centralized Model
All documentation is collected, reviewed, and managed by a single internal team—typically the sponsor or lead CRO.
- Pros: Greater control, consistency, and simplified oversight.
- Cons: May require additional internal resources for document handling.
Decentralized Model
Multiple stakeholders (e.g., sites, CROs, vendors) have direct access to contribute documentation to the eTMF.
- Pros: Reduces document handoffs and delays; encourages real-time updates.
- Cons: Requires robust permission management and audit trails.
Flex Databases supports both approaches by allowing highly configurable access permissions and workflows. Whether you want full control or a collaborative, multi-user setup, the platform adapts to your needs.
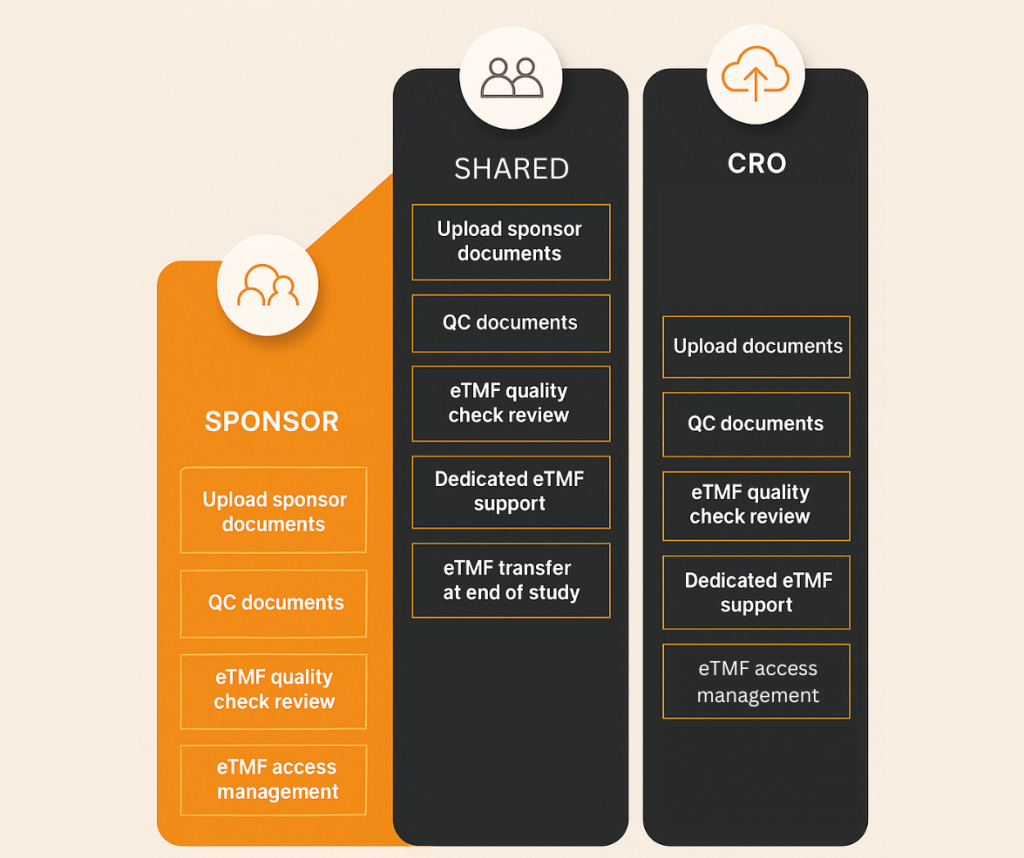
Sponsor-Controlled vs. CRO-Controlled vs. Hybrid Models
Sponsor-Controlled Model
The sponsor retains full ownership and management of the eTMF, even when working with CROs.
Best for: Sponsors who require full oversight and direct accountability.
CRO-Controlled Model
The CRO manages the eTMF on the sponsor’s behalf, including document uploads, quality checks, and completeness tracking.
Best for: Sponsors outsourcing full study execution.
Hybrid Model
Responsibilities are shared between sponsor and CRO, with clearly defined roles and permissions.
Best for: Collaborative partnerships with shared oversight.
Flex Databases easily accommodates all three setups with role-based permissions, user-specific document responsibilities, and audit logs that clearly identify document owners and reviewers.
Study-Based vs. Enterprise-Wide Architecture
Study-Based Model
Each study has its own isolated eTMF structure, allowing for tailored document sets and workflows.
- Pros: Flexibility and customization per trial.
- Cons: Less visibility across the portfolio.
Enterprise-Wide Architecture
A centralized eTMF system that spans all studies, programs, and stakeholders.
- Pros: Enables consistent processes, shared reference documents, and cross-study reporting.
- Cons: Requires upfront planning and governance.
Flex eTMF supports both configurations. Clients can start with a study-based approach and scale to an enterprise-wide system as their portfolio grows – without changing platforms or losing data continuity.
Flexibility Built In
Flex Databases offers modular, scalable, and compliant eTMF architecture that adapts to your existing processes rather than forcing you to change them. Whether you operate a biotech with limited trials, a CRO serving multiple sponsors, or a global sponsor managing dozens of studies, Flex provides the model flexibility, user control, and integration support to match your structure and scale.
Factors to Consider When Choosing an eTMF Model
Your choice should reflect the specific needs of your organization, your study types, and your operational workflows. Below are the key factors to consider, along with how Flex Databases helps you meet or exceed expectations in each area.
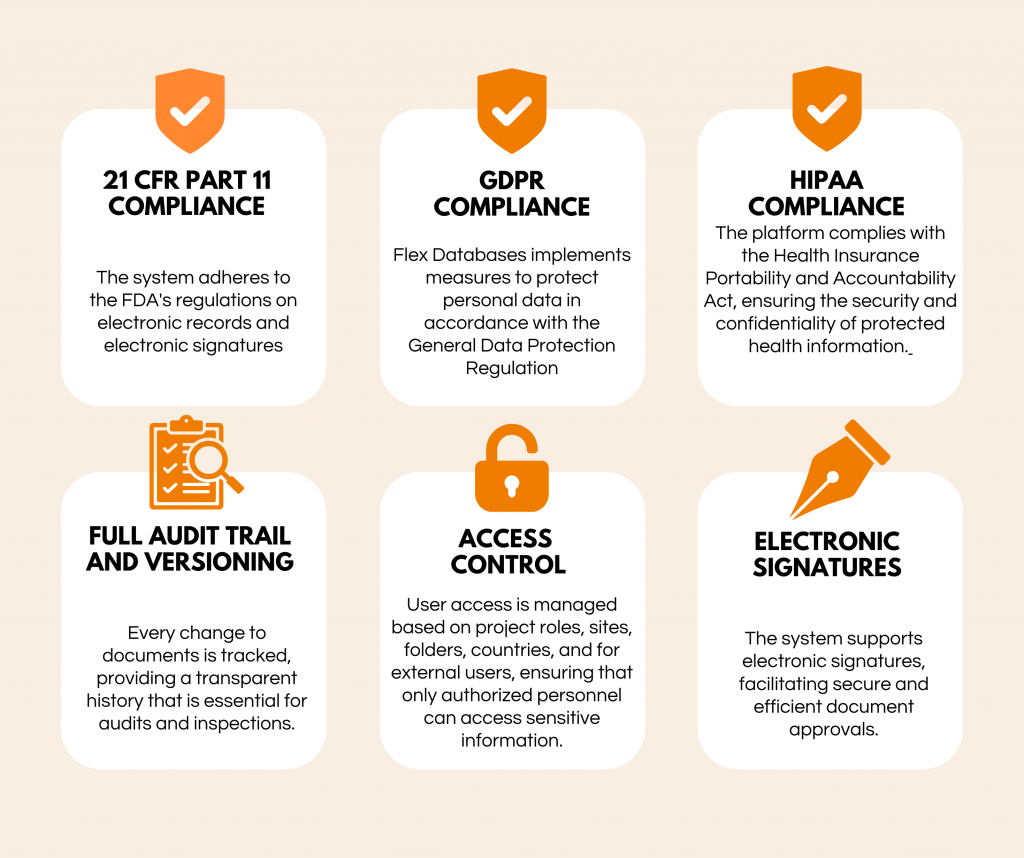
Compliance Requirements
Flex Databases’ eTMF is designed to meet key regulatory requirements:
- 21 CFR Part 11 Compliance: The system adheres to the FDA’s regulations on electronic records and electronic signatures, ensuring that electronic documents are trustworthy, reliable, and equivalent to paper records.
- GDPR Compliance: Flex Databases implements measures to protect personal data in accordance with the General Data Protection Regulation, safeguarding the privacy of trial participants.
- HIPAA Compliance: The platform complies with the Health Insurance Portability and Accountability Act, ensuring the security and confidentiality of protected health information.
Key features supporting compliance include:
- Full Audit Trail and Versioning: Every change to documents is tracked, providing a transparent history that is essential for audits and inspections.
- Access Control: User access is managed based on project roles, sites, folders, countries, and for external users, ensuring that only authorized personnel can access sensitive information.
- Electronic Signatures: The system supports electronic signatures, facilitating secure and efficient document approvals.
Study Portfolio Complexity
The complexity and size of your clinical trial portfolio will influence the ideal eTMF setup.
Whether you’re managing a single Phase I study or a portfolio of global, multi-site trials, Flex eTMF is designed to scale with your growth. The platform supports both standalone studies and integrated enterprise-wide operations, allowing you to start small and expand without disruption.
Integration Needs
Efficient document management depends on how well your eTMF connects with other systems.
Flex Databases offers native integration with its CTMS, Subject Tracking, and Investigator Site Management modules, ensuring seamless data flow. Additionally, robust API capabilities allow smooth integration with third-party EDCs, safety systems, or regulatory submission platforms – supporting true end-to-end trial oversight.
Team Structure and Ownership
Different stakeholders may need different levels of access and control over the eTMF.
Flex eTMF features highly configurable user roles and permission levels, supporting everything from sponsor-controlled models to collaborative environments involving CROs and sites. Document workflows, responsibilities, and approval chains can be tailored to reflect your team’s structure and SOPs.
Inspection and Audit Readiness
Regulatory inspections can happen at any time, so your eTMF must always be inspection-ready.
With real-time completeness tracking, automated document metadata capture, version control, and audit-ready dashboards, Flex eTMF provides continuous visibility into the state of your documentation. Review and QC tools ensure nothing is missed before, during, or after an inspection.
Budget and Resource Availability
Your technology investment should align with your operational maturity and financial resources.
Flex Databases offers modular pricing, allowing you to configure the eTMF based on your actual usage and requirements. Whether you’re a small biotech or a growing CRO, the platform provides cost-effective, scalable options to match your budget – without compromising functionality or compliance.
Real-World Scenarios
Flex Databases’ eTMF solution is flexible enough to meet the diverse needs of clients across different sizes and industries. Below are real-world examples of how clients use Flex eTMF to address their unique requirements:
Biotech Sponsor Running a Few Trials Per Year
A small biotech sponsor running only a few clinical trials annually often requires a streamlined and cost-effective solution that ensures full control over the process.
Solution: The sponsor opts for a simple centralized model where internal teams are responsible for all document management. This model allows for:
- Tight internal control over document uploads, quality checks, and approvals.
- Simplified user permissions to limit access to core team members.
- Full visibility into trial documentation status to ensure regulatory compliance without the need for complex workflows or external involvement.
Flex eTMF provides this sponsor with a straightforward, easy-to-manage solution that supports their operational scale while maintaining regulatory compliance.
Mid-Size CRO
A mid-sized Contract Research Organization (CRO) supporting multiple sponsors requires a flexible, multi-tenant system to handle the varied needs of their clients.
Solution: The CRO uses a multi-tenant setup with separate eTMFs for each client, which provides:
- Dedicated eTMF systems for each sponsor, ensuring that each client has a customized, isolated environment.
- Role-based access to guarantee the right stakeholders (sponsor, CRO team, sites) can access and manage relevant documents.
- Cross-study visibility for the CRO to manage timelines, track progress, and ensure that all clients are inspection-ready.
With Flex eTMF, the CRO can efficiently manage multiple clients and studies without sacrificing compliance, security, or operational efficiency.
Large Pharma
A large pharmaceutical company overseeing multiple, global trials requires a fully integrated enterprise-wide eTMF to unify document management across all departments, studies, and stakeholders.
Solution: The pharma company implements an enterprise eTMF architecture with full integration across various systems, including:
- Centralized document storage for easy access by internal teams, sites, and partners.
- Seamless integration with other platforms, such as CTMS and EDC, to ensure consistent data flow and eliminate silos.
- Global oversight of all trials with the ability to generate reports and track documents across studies and departments.
Flex eTMF offers this large pharmaceutical company a comprehensive, scalable solution that ensures cross-departmental coordination, compliance, and a single source of truth for clinical trial documentation.
Whether you’re a small biotech sponsor, a growing CRO, or a large global pharma, Flex eTMF adapts to your unique operational needs, and ensures you can manage your trials efficiently and stay inspection-ready at all times.

How to Choose the Right Model with Flex Databases
At Flex Databases, we guide you through every step to build a solution that fits best with your operational structure. Here’s how we help you choose the right model:
Start with a Discovery Call with Our Product Experts
We begin by organizing a discovery call with our product experts to gain a deep understanding of your unique requirements. During this session, we will:
- Discuss your business objectives and specific trial needs.
- Identify any challenges you are currently facing with document management.
- Ensure alignment between your goals and our eTMF capabilities.
This collaborative approach ensures we understand the full scope of your needs before recommending a solution.
Map Your Current Processes and Challenges
Our team works with you to map your current processes, from document submission to approval workflows. Together, we’ll:
- Analyze how documents are currently managed across your trials.
- Highlight gaps, delays, or potential regulatory issues in your current workflows.
- Understand the challenges you face, including cross-team collaboration, data consistency, and trial oversight.
This in-depth mapping allows us to identify the best eTMF model to optimize your existing processes.
Define Access Levels, Integration Points, and Reporting Needs
To ensure seamless integration and secure access management, we work closely with you to define:
- Access levels: Who needs access to which documents, and what permissions should be set for each role (sponsor, CRO, site, etc.)?
- Integration points: Whether you require integration with CTMS, EDC, or other third-party systems, we determine the necessary touchpoints.
- Reporting needs: We identify the key metrics and reports you need to generate, whether it’s for trial progress, audit readiness, or regulatory compliance.
This ensures that the eTMF will align with your operational workflow and strategic objectives.
Build a Custom Configuration That Fits Your Model – Not the Other Way Around
At Flex Databases, we believe that the technology should adapt to your needs, not the other way around. Based on the insights gathered from the workshop, process mapping, and requirements definition, we build a custom configuration that:
- Fits your trial model: Whether centralized, hybrid, or enterprise-wide, we create a solution that works for your organization.
- Scales with your needs: As your company grows and evolves, your eTMF can be adjusted to fit new requirements.
- Ensures full compliance: We tailor the configuration to maintain compliance with global regulatory standards.
With Flex Databases, you have a partner who is committed to building a customized solution that aligns with your unique operational needs and goals.
Mistakes to Avoid
When selecting and implementing an eTMF model, certain missteps can lead to inefficiencies, user frustration, and compliance issues. Here are the most common pitfalls – and how Flex Databases helps you avoid them:
One-Size-Fits-All Model
Not all organizations operate the same way. Selecting a rigid, standardized eTMF model without considering your unique processes, team structure, and study complexity can limit flexibility and long-term scalability.
With Flex Databases, your eTMF configuration is fully customized to match your operational model – whether you’re a sponsor, CRO, or somewhere in between.
Underestimated Integration Challenges
Ignoring the importance of seamless integration with other systems – such as CTMS, EDC, or safety databases – can create data silos, duplicate work, and increase the risk of inconsistencies.
Flex eTMF offers native integration with other Flex modules and robust API capabilities to support smooth data flow across platforms.
Lack of Attention to User Adoption
Even the best-configured eTMF system will underperform if users don’t understand how to use it effectively. Lack of onboarding and ongoing support can lead to low adoption and compliance gaps. Flex Databases provides:
- Comprehensive user onboarding
- Ongoing client support
- Tailored system training
This ensures your team is confident and your processes remain consistent and compliant from day one.
With Flex Databases, you’re not just getting a system – you’re getting a partner dedicated to building the right solution for you.
Ready to explore the best eTMF model for your organization?
Book a call with our experts today at contact@flexdatabases.com to get started.



