Risk-Based Monitoring with Flex Databases
October 1, 2025

Flex Databases combines deep clinical expertise with advanced technology to deliver a smarter approach to trial oversight. Our Risk-Based Monitoring (RBM) framework helps sponsors and CROs focus on what matters most, reduce effort and cost, and make faster, data-driven decisions – supported by Flex Databases’ EDC, central/remote monitoring, and AI analytics.
Why Risk-Based Monitoring?
- Focus effort on critical risks and problem areas
- Fewer errors and protocol deviations
- Lower monitoring costs without sacrificing quality
- Higher-quality analysis and insights
- Data-driven decisions based on statistics and AI
- Consistent cross-site comparisons
- Faster, more reliable outcomes
How it works
- Risk identification & assessment → define critical processes and potential vulnerabilities
- Central & remote monitoring → RBM dashboards, statistical tools, and AI analytics in Flex EDC
- Targeted SDV → verify only where risk justifies it
- Continuous oversight → early anomaly detection and corrective/preventive actions
- Close-out insights → evaluate RBM effectiveness to optimize future studies
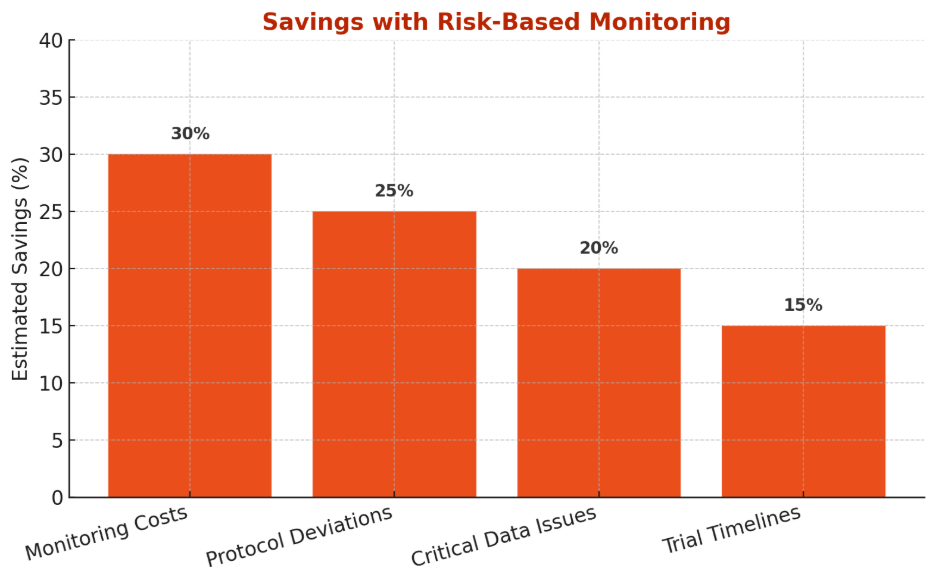
Where you save with RBM
The chart below highlights the primary areas where RBM delivers tangible efficiencies
Smarter Trials, Stronger Outcomes
Risk-Based Monitoring is more than a methodology – it’s a transformation in how clinical trials are managed. With Flex Databases, you gain a partner who combines innovation, regulatory expertise, and proven delivery to help you run trials more efficiently, reduce risks, and bring life-changing medicines to patients faster.



