Flex Databases Security Policy Overview
Flex Databases Platform is designed to help our Clients to work securely with their data and deliver it to users, customers, and partners, and to drive better business outcomes. Flex Databases protects Clients’ data, ensure the following security regulations, and mitigates all potential risk which is essential to building trust and delivering the high level of service. Flex Databases takes a risk-based approach to security, and this page details the measures and technologies implemented to protect our customers.
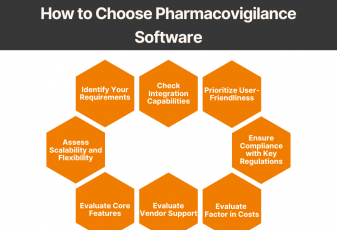
Our security implementation allows us to adhere to the following standards and best practices, demonstrating our commitment to customers’ security and privacy:
As a SaaS provider Flex Databases pays great attention to the safety of the data on all levels. We use only the best scenarios and industry practices to keep and protect customers’ data: from physical security at the data centers to full control using audit trail at the data level:
- Processes (SOPs, IQs, PQs, WPs)
- Physical
- Infrastructure
- Virtual environment
- Application
- User
- Data
We have a strict backup policy:
To maintain a robust disaster recovery strategy, backups are retained at separate Flex Databases data centers at a geographically different location within the same region as the primary data center, with the same level of physical and infrastructure security described above, to maintain a robust disaster recovery strategy. All backups are encrypted-in-transit to the separate data center and are encrypted-at-rest while stored at that location. We are in the total charge for the security of data; that’s why we use the best experience in data storage and backup technology.
- We use GFS (grandfather-father-son) rotation strategy.
- We do an incremental backup of all client’s data two times per day, including weekends.
- We perform FULL back up every day, including weekends.
- We keep data for the last two weeks, for the previous two weeks, per each month of the year and per each year all the time.
If you have any questions, regarding our security policy or any other questions, send us an e-mail at contact@flexdatabases.com