TMF Quality Review 2.0
January 25, 2024
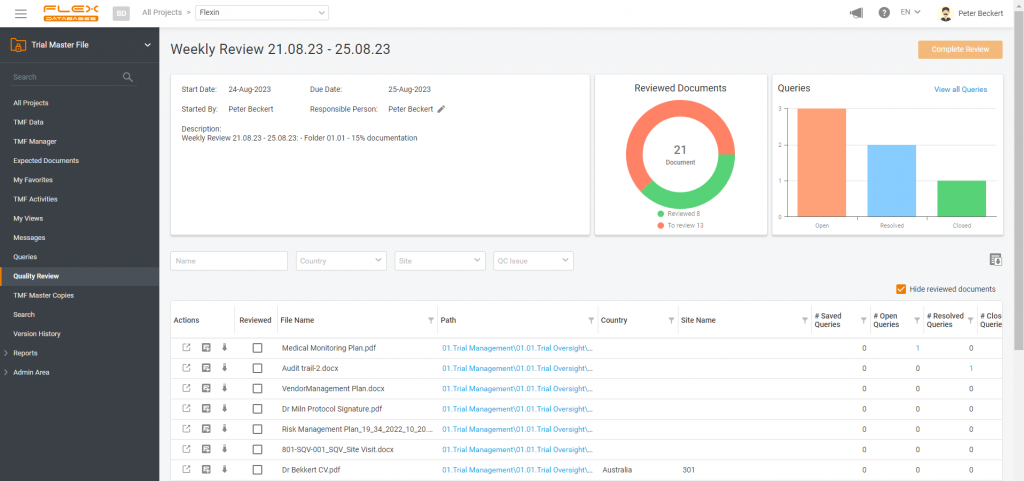
Periodic TMF reviews automatically documented. Quality and completeness under control!
Let’s address an intriguing issue in the clinical trial landscape. It appears that while one third of sponsors are less than satisfied with the quality of clinical trials, an overwhelming 90% of CROs are confident in their high-quality delivery. The solution might lie in implementing transparent, easily trackable KPIs through systems like CTMS and eTMF.
Tracking and checking can be time-consuming, but this is where the automated quality review function within eTMF shines. It streamlines the process, allowing for:
- Demonstrated compliance and oversight for inspections
- Effortless reporting on TMF status both internally and to sponsors
While compliance is common among most companies, the real challenge lies in demonstrating this compliance. It’s crucial not just to adhere to the standards but also to effectively showcase it, whether it’s from CROs to sponsors, or from sponsors to inspectors.
We have a solution: automatically generated evidence of periodic TMF reviews. Set up the review schedule in the system, and it takes care of the rest – reminders, comprehensive document, and folder checks, recording all queries and resolutions, and then generating a detailed report. This makes sponsor oversight transparent and well-documented.
Enhanced Quality Review function allows for scheduled periodic reviews, selection of specific documents for review, assignment of reviewers, and progress tracking. Every step, from document checks to folder completeness, is recorded, and displayed in the system. Once the review is complete, a report on the periodic check is automatically generated, facilitating an easy demonstration of thorough oversight.
Get in touch for a demo on top of the page or just email us at bd@flexdatabases.com.



