How to Set Up an Effective QMS
August 15, 2024
Clinical trials provide essential information on the safety and effectiveness of drugs. Sponsors ensure that investigators follow protocols and collect and report quality data. Quality in clinical trials means that the product or service meets applicable regulations and customer requirements. A Quality Management System (QMS) in clinical research ensures compliance with ethical, regulatory, and GxP standards while maintaining the authenticity of clinical data. The main parts of a QMS in clinical research are quality control and quality assurance.
What is the Quality Plan?
The quality plan outlines how quality control and quality assurance processes are followed during the clinical trial. It describes various quality-related tasks and documents specific practices, resources, and activities for the project. We at Flex Databases are always ready to sign the Client-specific Quality Plan to capture our commitments.
Quality Control and Quality Assurance Activities in a Clinical Trial:
- Ensure compliance with Protocol, Standard Operating Procedures (SOPs), and Good Clinical Practice (GCP). (Flex Databases LMS allows to assign general and project-specific training followed by the quizzes in the System and follow up on them)
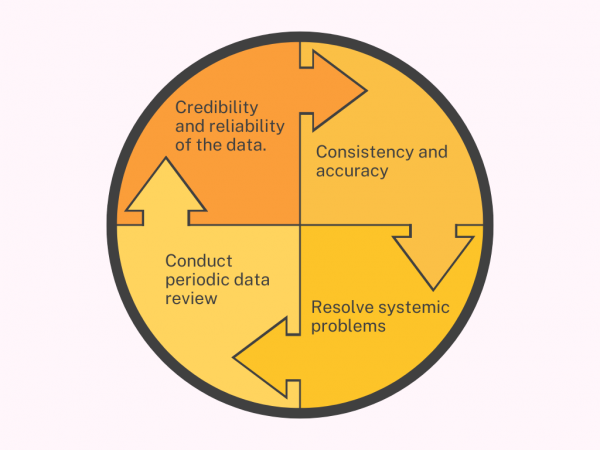
- Resolve systemic problems before the trial ends.
- Resolve data queries.
- Find ways to reduce cycle times for different processes.
- Ensure the credibility and reliability of the data.
- Maintain consistency and accuracy of data at all stages.
- Address cases of nonconformity during the trial
- Conduct periodic data review (Flex Databases TMF enables quick and easy TMF Quality reviews)
- Conduct audits (you can plan, report and follow up them using Flex Databases QMS module!).
Quality System Structure
A strong clinical trial system includes:
- Clear Written Procedures: Have well-documented procedures to ensure the system works correctly.
- Defined Roles and Responsibilities: Ensure everyone knows their roles, responsibilities, and tasks.
- Control and Review: Regularly review and update the system as needed.
- Defined Standards: Follow established standards like ICH-GCP and European Directives.
Written procedures can include:
- Policies: Top-level documents that outline key system goals.
- Standard Operating Procedures (SOPs): Documents describing actions for specific situations.
- Work Instructions (WIs): Detailed instructions for performing tasks.
- Forms, Templates, and Records: Evidence of following procedures.
The complexity of the quality system varies based on the organisation. Large global pharmaceutical companies or CROs may use a hierarchical approach with these documents to manage their quality systems effectively.
Key Success Factors for an Efficient QMS
How to ensure your SOPs are compliant and used by your staff:
- Make SOPs User-Friendly: Design them to be easy to understand and navigate to support compliance.
- Consult with Staff: Interview senior leaders and staff to outline each process and confirm responsibilities. Use their input to draft or update SOPs, then review and approve them.
- Balance Length with Detail: For complex processes, like Sponsor’s Clinical Oversight, consider creating an SOP with separate work instructions and forms rather than a lengthy single document.
- Use Visuals: Include charts and diagrams in your SOPs to explain complex information and make it easier to understand.
- Perform a Gap Analysis: Regularly check if your SOPs cover all activities and comply with regulations, regardless of when your QMS was established.
- Adapt for Local Needs: Ensure global procedures can be adjusted to meet local regulations.
- Control Document Versions: Keep track of the latest approved versions of your SOPs through version control, distribution control, and access control.
Conclusion
A well-organised and easy-to-use QMS is crucial for successful clinical trials. It ensures you follow regulations, clearly define roles and responsibilities, and adapt to local needs. To build an effective QMS, use user-friendly procedures, include visual aids, and perform gap analyses. Always focus on compliance, staff training, and keeping procedures updated with version control. A strong QMS helps organisations maintain high quality standards in their clinical trial activities.
Flex Databases offers a QMS module that will help QA professionals to have all their tasks completed in one place, including CAPAs, incidents, change management and QMS documents. Without any additional efforts and applications the module allows:
- Create a new QMS document or initiate review of the existing one
- Review QMS documents with comments and revision history stored in the System
- Get notifications regarding periodic review scheduled
- Finalise, sign and issue the document within the System
- Assign the training and the quizzes, check the results and don’t miss the due dates.



