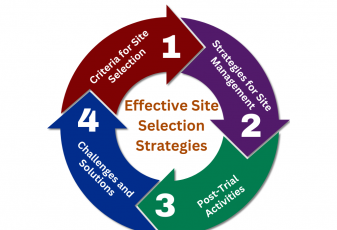
Effective site selection and management are critical components of successful clinical trials. Choosing the right locations and overseeing their operations can significantly impact the trial’s timeline, budget, and ultimately, its success in delivering reliable data. This article explores essential strategies for optimizing site selection and management, highlighting key considerations, practical tips, and the role of […]