Top 10 Benefits of Modern CTMS for CRO’s
June 25, 2024
Clinical Research Organizations (CROs) operate in a dynamic industry where efficiency and adaptability are critical. As the global clinical trials market expands significantly, with forecasts predicting substantial growth, CROs face increasing demands to manage multiple trials efficiently across diverse projects and stakeholders.
Legacy systems, still prevalent among CROs according to recent surveys, often fall short in meeting these complex needs. They struggle to provide the flexibility, integration capabilities, and intuitive user experience required to streamline operations effectively. In contrast, modern Clinical Trial Management Systems (CTMS) like Flex Databases CTMS offer tailored solutions that significantly enhance CRO performance and client satisfaction.
Key Benefits of Switching to Flex Databases CTMS
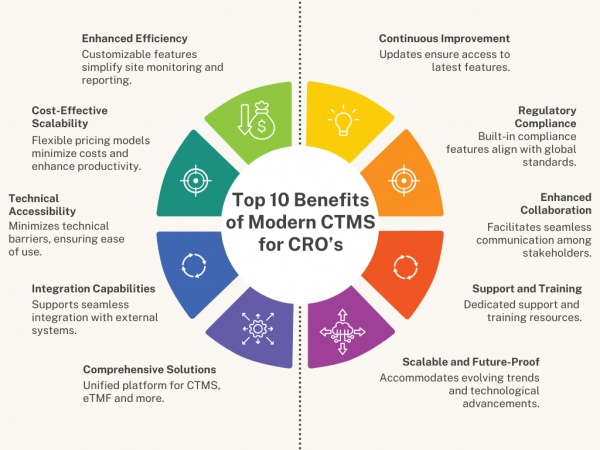
- Enhanced Efficiency: Flex Databases CTMS simplifies site monitoring and reporting with customizable features that empower users to create detailed, sponsor-specific reports independently. This autonomy improves workflow efficiency and responsiveness.
- Cost-Effective Scalability: Flexible pricing models allow CROs to scale operations according to trial demands, minimizing costs while maintaining operational agility. Quick onboarding and intuitive interfaces further reduce training time and enhance productivity.
- Technical Accessibility: Flex Databases CTMS for CRO’s minimizes technical barriers, ensuring accessibility and ease of use. This approach accelerates deployment and reduces the risk of operational delays.
- Integration Capabilities: our CTMS supports seamless integration with various external systems through standard APIs, facilitating cohesive data management across platforms. This versatility ensures compatibility with existing IT infrastructures.
- Comprehensive Solutions: As an all-in-one platform for CTMS, eTMF, and more, CTMS offers a unified solution that eliminates the need for multiple disparate systems. This integration enhances data oversight and operational efficiency.
- Continuous Improvement: With constant updates and enhancements delivered seamlessly through SaaS deployment, Flex Databases CTMS adapts to evolving industry demands and client needs. This commitment to innovation ensures CROs always have access to the latest features and improvements.
- Regulatory Compliance: With built-in compliance features aligned with global regulatory standards such as ICH-GCP, Flex Databases CTMS helps CROs ensure adherence to regulatory requirements throughout the trial lifecycle. Comprehensive audit trails and document management support further streamline compliance efforts.
- Enhanced Collaboration: Facilitating seamless communication and collaboration among stakeholders, Flex Databases CTMS improves coordination between CROs, sponsors, and clinical sites. Features like secure document sharing and task assignment enhance team efficiency and project transparency.
- Support and Training: Flex Databases CTMS offers dedicated customer support and training resources to assist CROs throughout the implementation and operational phases. This proactive support ensures smooth adoption and maximizes the system’s benefits.
- Scalable and Future-Proof: Designed to accommodate evolving industry trends and technological advancements, Flex Databases CTMS is scalable and adaptable. Regular updates and enhancements ensure that CROs always have access to the latest features and improvements, future-proofing their trial management processes.
Flex Databases CTMS stands out as the optimal choice for CROs seeking a modern, comprehensive solution to streamline clinical trial management. By embracing our CTMS, CROs can elevate their operational capabilities, enhance client satisfaction, and maintain a competitive edge in the rapidly evolving clinical research landscape.



