World Backup Day – March 31st!
March 29, 2019
March 31st is important for every data related human – World Backup Day.
World Backup Day was established by enthusiasts to remind everyone about the importance of keeping personal data safe.
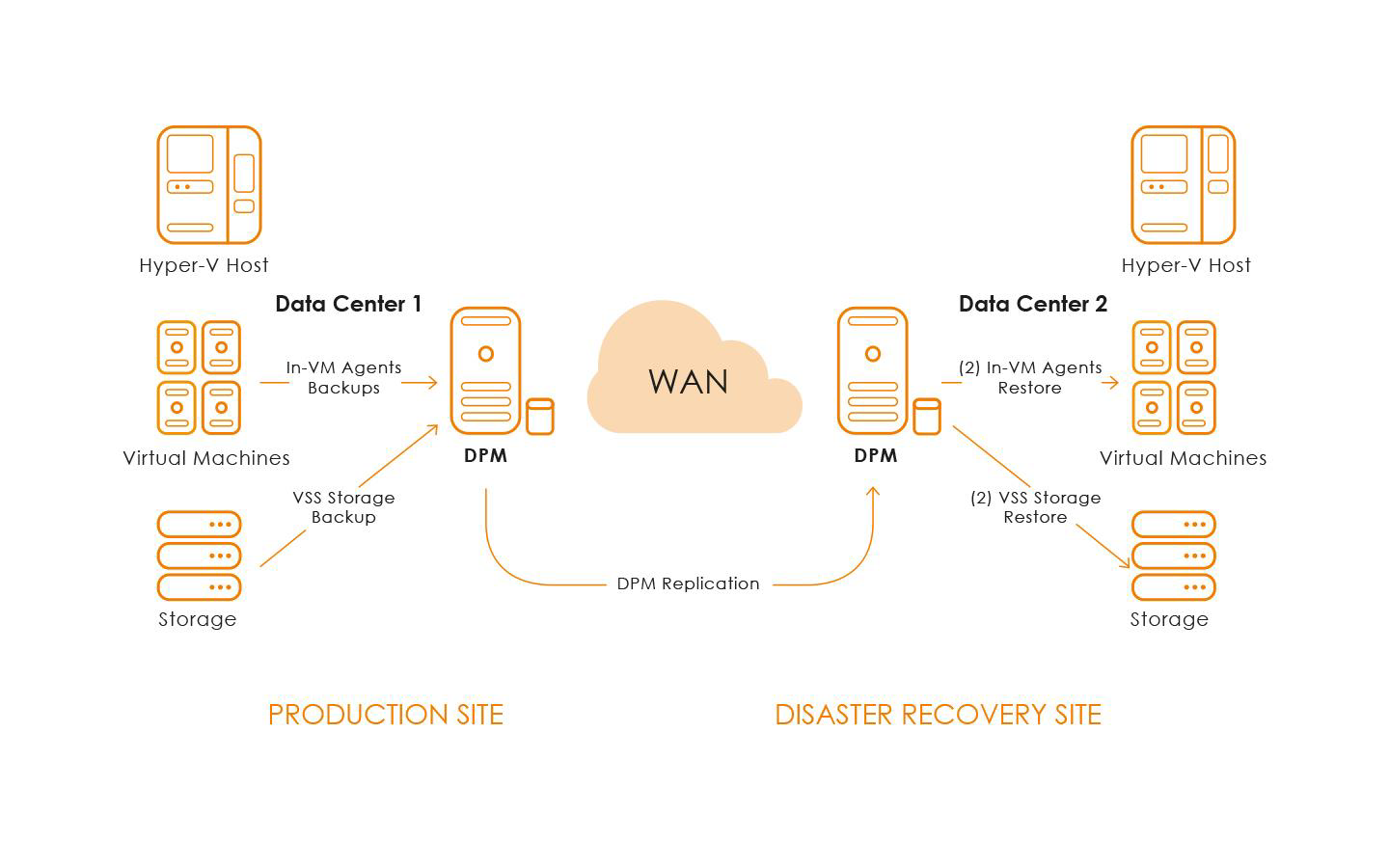
enters at a geographically different location within the same region as the primary data center, with the same level of physical and infrastructure security described above, to maintain a robust disaster recovery strategy. All backups are encrypted-in-transit to the separate data center and are encrypted-at-rest while stored at that location. We are in total charge for the security of data, that’s why we use the best experience in data storage and backup technology.
- We use GFS (grandfather-father-son) HDD rotation strategy.
- We do incremental backup of all client’s data two times per day including weekends.
- We perform FULL back up every day including weekends.
- We keep data for the last two weeks, for the previous two weeks, per each month of the year and per each year all the time.
- Each Monday we deposit encrypted backup in the Bank safe (out of data center).
Have any questions about our backup policy? Send us an e-mail to contact@flexdatabases.com
Stay safe and have a Happy World Backup Day!



