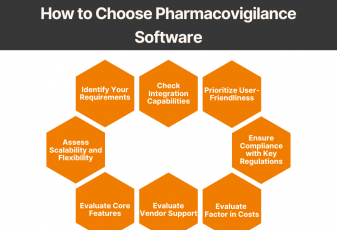
Stay Compliant, Stay Confident
In today’s regulatory landscape, software validation is not just an option — it’s a necessity. Validated software is a key requirement, mandated by regulatory bodies like the WHO Expert Committee on Specifications for Pharmaceutical Preparations and the EMA Guideline on computerized systems and electronic data in clinical trials.
At Flex Databases, we understand the importance of compliance and accuracy in clinical trials. Our validation process ensures that all computerized systems used within a clinical trial consistently meet specified requirements, ensuring reliability and intended performance throughout the product’s lifecycle.
Validation as a service
We happily apply our hassle-free, quality-driven validation experience to support our clients. Our expert team follows best practices, aligning with regulatory guidelines. The validation methodology is based on GAMP® 5 guidance. We employ an Agile & SCRUM approach, frequently updating our systems to keep pace with industry trends and regulatory changes.
Validation deliverables
We provide all the necessary documentation to be included in the Client’s validation package:
- Validation Summary Report for all our validation efforts as system developer
- User Requirements Specifications
- OQ Testing Summary Report
- User Manual
- Release Notes
- IQ documentation
- Maintenance Plan
- Training Certificates
- And we do provide the necessary documents ensuring you are aware of and control all the changes implemented into the system after the first release to production.
Your Success Is Our Priority
As your trusted partner, we offer comprehensive services and deliverables for streamlined GxP-regulated system validation.
- Formalized User Requirements Specification (URS) compliant with 21 CFR Part 11 for e-records and e-signatures
- Deployment of Client’s private cloud instance with Installation Qualification (IQ) documentation
- Training in accordance with our Quality Management System, industry standards, and applicable regulations
- Updated validation documents with every new system version release, adhering to risk-based approaches recommended by industry standards
Global Reach, Top Performance
Our data centers, located worldwide, are handpicked from an approved vendor list. This strategic approach not only ensures regulatory compliance but also guarantees stable performance. For EU-based clients, we offer data centers in Germany and Finland, and US centers for our American clients. All the centers have successfully passed the vendor-assessment audits and comply with personal data protection regulations.
Let’s Accelerate Your Validation Journey
We believe in making the validation process smooth and efficient for our clients. Our team is ready to guide you through the system during your user acceptance testing (UAT), providing the necessary documents to ensure your success.
Your Audit-Ready Partner
Flex Databases’ Quality Management System (QMS) covers all processes, supporting vendor assessment with a comprehensive range of documents, ready for your audit needs. We’ve successfully passed numerous external audits by esteemed international CROs, pharmaceutical companies, and independent auditors.
Reach out to contact@flexdatabases.com to embark on a seamless validation journey with Flex Databases.
Learn more about regulatory recommendations regarding validation with these valuable resources: