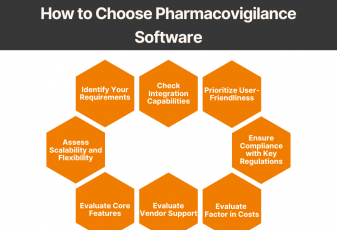
Pharmacovigilance (PV) is a critical aspect of drug safety, focusing on detecting, assessing, understanding, and preventing adverse drug reactions or other drug-related problems. As global regulatory requirements for drug safety evolve, adopting reliable pharmacovigilance software has become essential for ensuring compliance and improving operational efficiency. Selecting the right PV software can significantly impact your organization’s […]