Blog Flex Databases
BIOTRONIK, a leading global medical device company, has selected Flex Databases as its trusted eClinical partner to streamline and enhance the management of its clinical trial operations through Flex Databases’ unified platform. Comprehensive Functionality and Strong ROI Following a rigorous evaluation process, BIOTRONIK chose Flex Databases for its broad functionality, competitive pricing, and exceptional return […]
Our Head of Business Development and Marketing, Marietta Sarkisian, sat down with Ajay Sadhwani, Senior Vice President at Harbor Clinical, to talk about their experience with Flex Databases Pharmacovigilance module. In this conversation, Ajay shares how smooth implementation, responsive customer support, and the flexibility of our PV system helped Harbor Clinical streamline operations and enhance […]
Dana Leff Niedzielska, MBA, SVP of Global Clinical Operations at Everest Clinical Research, shares her experience using Flex Databases’ CTMS and eTMF. In this interview, Dana highlights how Flex Databases supports Everest CRO’s clinical operations. Learn why Everest chose Flex Databases as their trusted technology partner and why they continue to rely on and trust […]
About SOLTI SOLTI is a non-profit academic research group specializing in breast cancer clinical and translational research. Based in Barcelona, the group unites over 510 professionals across 113 hospitals, including oncologists, pathologists, radiologists, and study nurses. With 42 ongoing studies and a team of 60+ staff members, SOLTI supports clinical trials throughout their entire lifecycle […]
At Flex Databases, we believe the best solutions are built together. That’s why we created Flex Databases User Groups – a unique meetings where our clients and their end-users come together to: Our User Groups are more than just meetings – they are a space for open dialogue, collaboration, and innovation.By listening to your experiences, […]
Alithia Life Sciences is a full-service Contract Research Organization dedicated to deliver high-quality clinical trial management solutions. With a strong focus on efficiency, compliance, and operational excellence, Alithia partners with pharmaceutical and biotech companies to ensure the seamless execution of clinical trials. To further enhance its document management and trial oversight, the company selected Flex […]
Palobiofarma is a leading clinical-stage biotech company dedicated to developing innovative medicines. Our focus on adenosine signaling drives our commitment to scientific excellence, with six drug candidates currently in different phases of development. Among them, PBF-999 for Prader-Willi Syndrome stands out as a key project. Our mission is to advance patient care by leveraging cutting-edge […]
October, 2024 – Agenus Inc. (NASDAQ: AGEN), an immuno-oncology company with a strong pipeline of immune modulating antibodies, together with Flex Databases announce the successful implementation of Flex Databases’ Clinical Trial Management System (CTMS) and electronic Trial Master File (eTMF) solutions, including a new AI-driven feature for automated document filing and metadata assignment. This partnership […]
We had the pleasure of sitting down with Martin Krauss, Co-Founder and Managing Director of FGK CRO, to discuss how Flex Databases has supported their clinical research operations over the past 3.5 years.In this interview, Martin shares: • Why FGK CRO initially chose to partner with Flex Databases.• How our CTMS, TMF, and LMS solutions have transformed […]
Metronomia, a leading Data Management and Biostatistics CRO in EU, has selected Flex Databases’ eTMF module to support one of its biotech clients. Simon Rückinger, Managing Director at Metronomia Clinical Research GmbH, explained their decision and what they expect from the partnership. “We needed a flexible and simple eTMF solution that could meet the specific […]
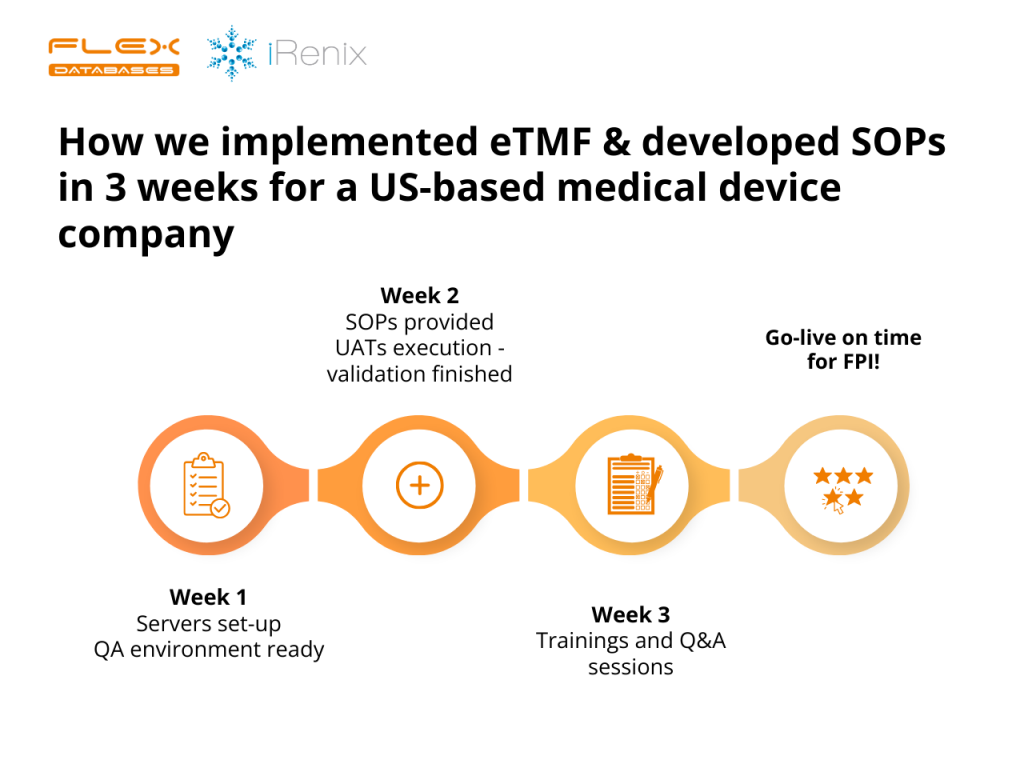
Last year, right around this time, we implemented an eTMF for a California-based Medical Device company, iRenix. Our target? A swift 3-week completion, so everything was up and running before their FPI. Now, this is quite the sprint compared to the usual 5-6 weeks! And here’s the twist – while iRenix was laser-focused on their […]
Deloitte, a renowned leader in professional consulting, and Flex Databases, a trailblazer in flexible electronic systems, have officially announced their strategic alliance, signed at the end of year 2023. This collaboration is set to transform the Life Sciences sector by leveraging Deloitte’s expertise in process management, validation, onboarding, and consulting, alongside Flex Databases’ state-of-the-art electronic […]