On the first day of June we’ve hosted a joint webinar with our client, Confidence Pharmaceutical Research.

On the first day of June we’ve hosted a joint webinar with our client, Confidence Pharmaceutical Research.

In early April 2023 we’ve hosted a webinar about why and how you can digitalize your Quality Management System with our brilliant QA Director Anna Petrovskaya as a host.
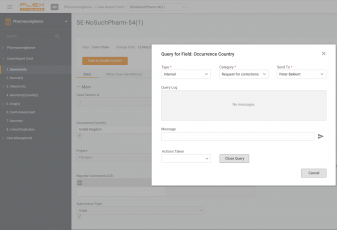
In this video we talk about the way Flex Databases Pharmacovigilance module helps to save time on case intake.

A detailed overview of software validation process in clinical trials industry

The system owner is the client’s employee responsible for the overall implementation and operation of a clinical trial management system on the client’s side.
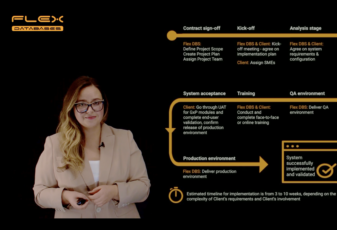
We can deliver a fully functional system in the timeframe from 3 to 10 (typically around 5) weeks, depending on the Client’s involvement and the complexity of the Client’s requirements.

What are the components of a typical eClinical software pricing and how do we at Flex Databases approach it? Here’s an answer.

In this video, we’ll tell you about Flex Databases CTMS & eTMF implementation for FGK CRO.
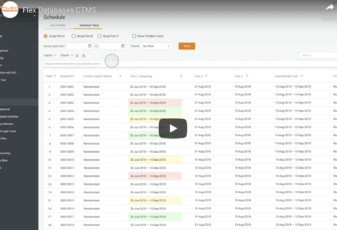
Flex Databases Platform: Interface Overview
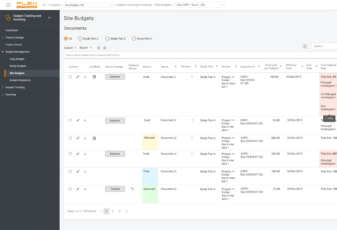
Use Subject Tracking & Invoicing Module to track all patients’ data and manage invoicing online. Plan and schedule patient visits efficiently.
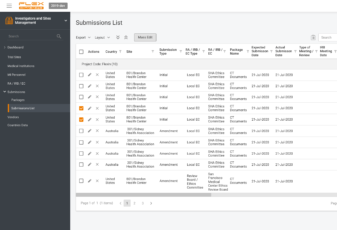
Investigators & Sites Management Module helps to organize all information on investigators, sites, vendors, therapeutic areas, IRB/LECs, etc. It provides easy search capability for feasibility assessments and other needs.
Flex Databases CTMS – CRA Activity Management