




One system for clinical development process and beyond. Evidence-based decisions. More information – less efforts.





Drop us a line and we be happy to answer all your questions
Clients
Daily users
Documents
Clinical trials
Countries
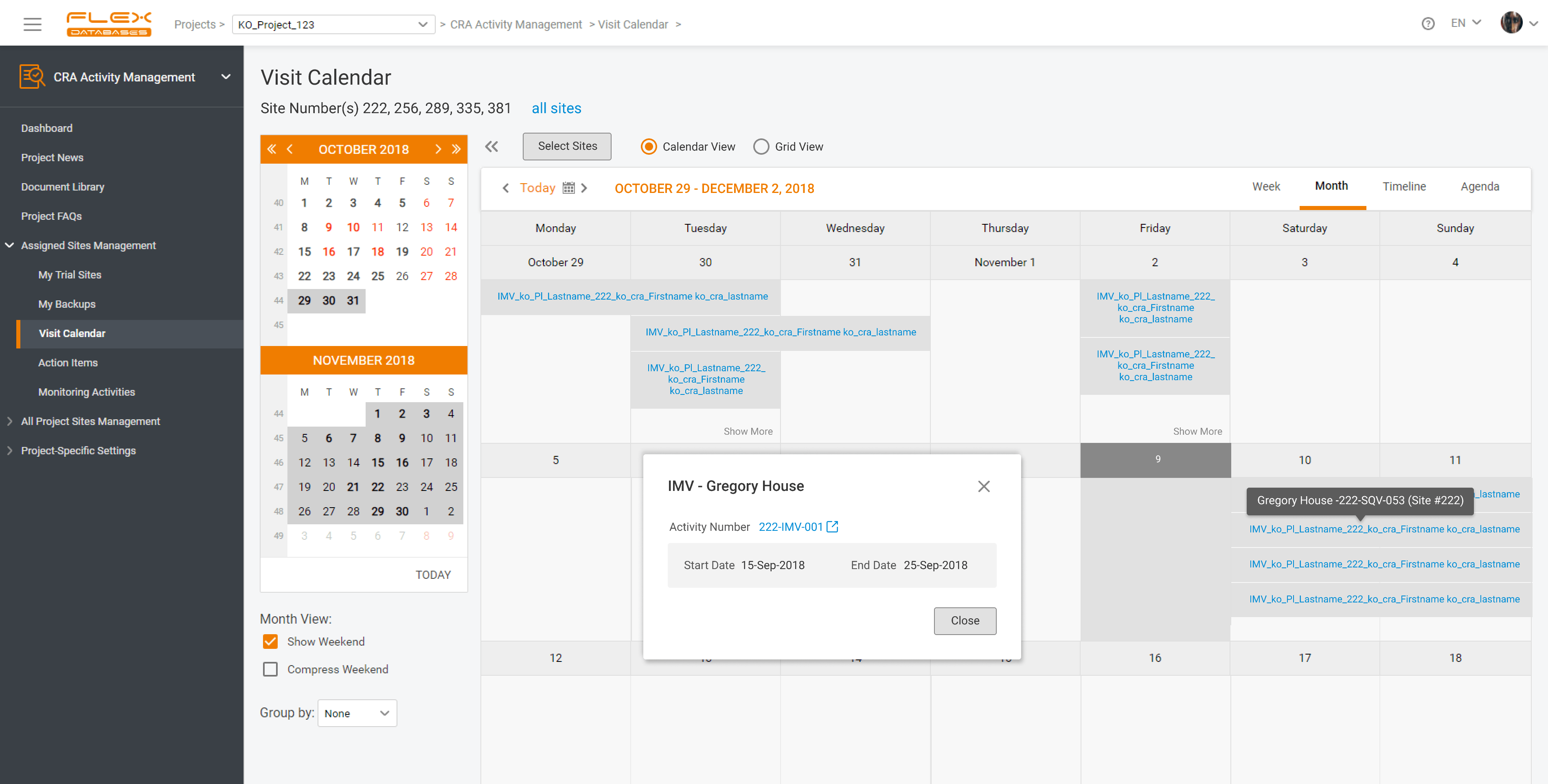
What is CTMS A CTMS – Clinical Trial Management System is a software solution that facilitates more streamlined management and monitoring of clinical trials. Clinical operations teams like project managers, CRAs, and study coordinators in pharmaceutical firms, CROs, and biotech firms use the tool to oversee everything from site selection and subject recruitment to monitoring […]
About SOLTI SOLTI is a non-profit academic research group specializing in breast cancer clinical and translational research. Based in Barcelona, the group unites over 510 professionals across 113 hospitals, including oncologists, pathologists, radiologists, and study nurses. With 42 ongoing studies and a team of 60+ staff members, SOLTI supports clinical trials throughout their entire lifecycle […]
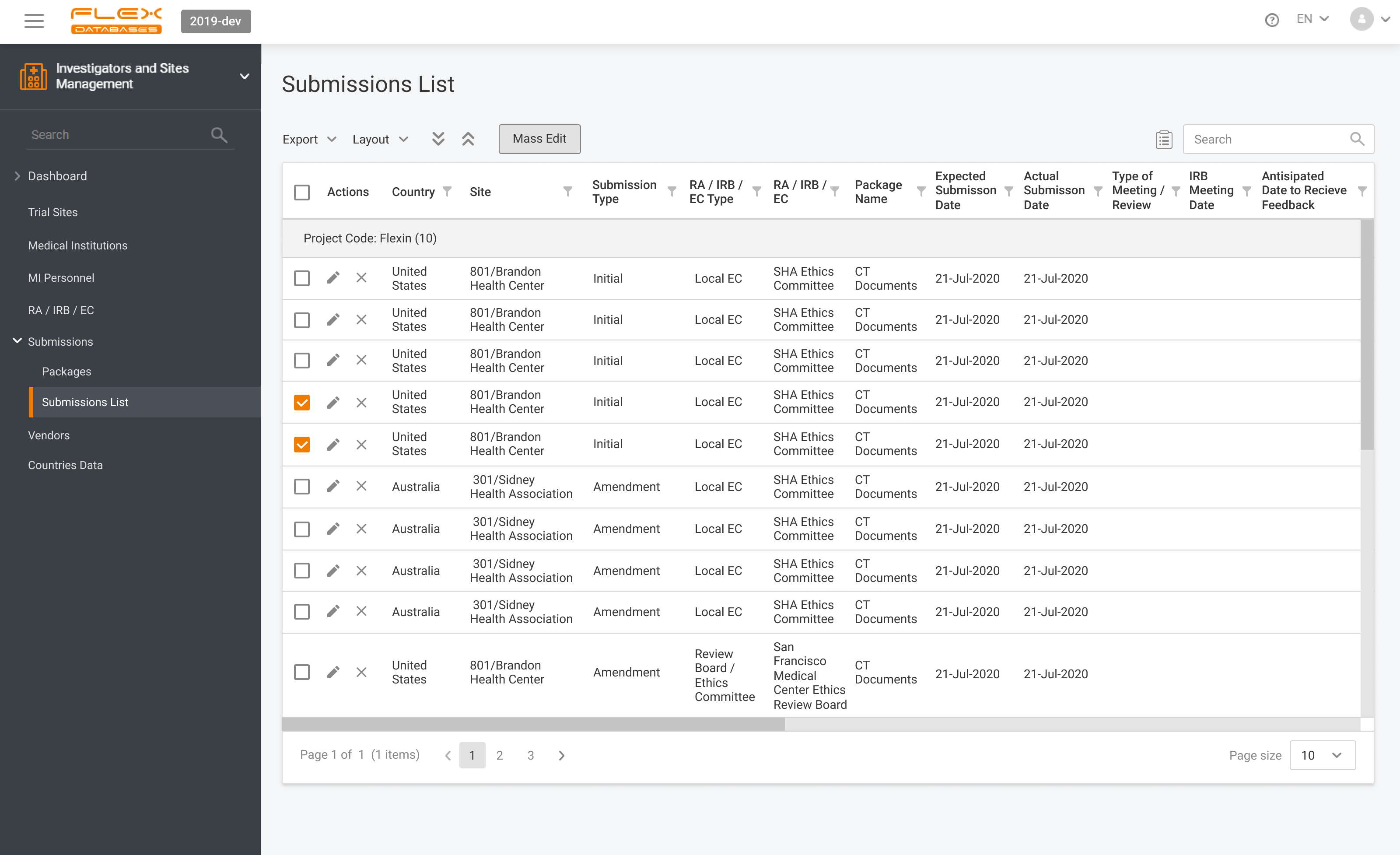
Nowadays the relationship between sponsors and contract research organizations (CROs) has evolved. What once was a simple outsourcing arrangement has become a strategic partnership, where both parties are expected to operate with full transparency, speed, and strict adherence to regulatory standards. Sponsors are no longer satisfied with periodic updates and disconnected spreadsheets. They want real-time […]
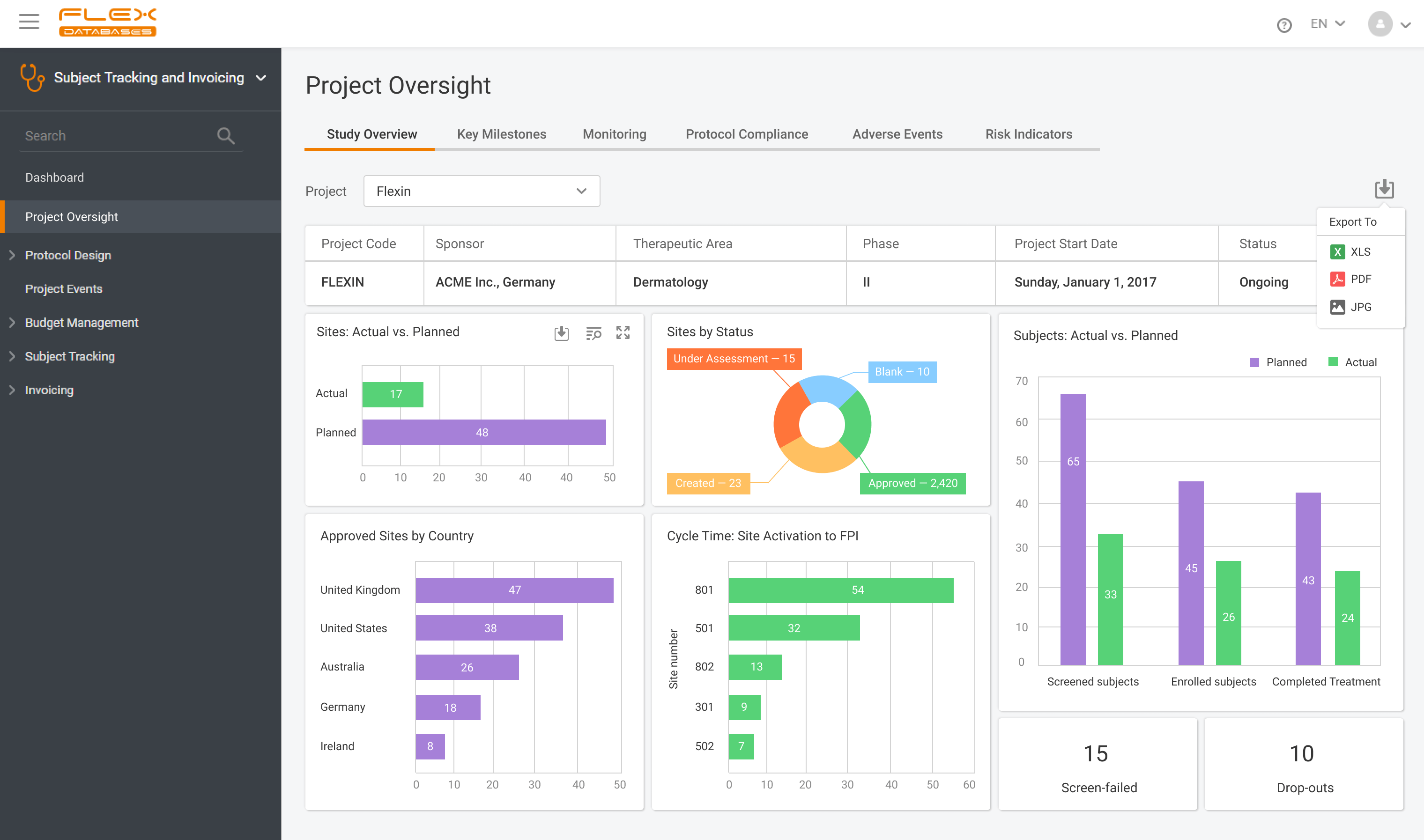
Clinical trials are essential for developing new medical treatments, yet they often face challenges like complex protocols, regulatory compliance, and data management issues. Implementation of a Clinical Trial Management System (CTMS) can address these challenges by centralizing data, automating workflows, and ensuring compliance, thereby enhancing efficiency and reducing errors. The adoption of CTMS solutions is […]
Get in touch to discuss compliance, implementation, demos, pricing
We are here for all of your questions! Tell us more about yourself and we will organize a tailored live demo to show how you can power up your clinical trials processes with Flex Databases.