Difference Between CDMS and CTMS
August 1, 2024
Clinical Data Management Systems (CDMS) and Clinical Trial Management Systems (CTMS) are two key tools that play crucial roles in this process. While they might sound similar, they serve distinct purposes and offer different functionalities.
What is a Clinical Data Management System (CDMS)?
A Clinical Data Management System (CDMS) is a specialised tool designed to manage and oversee the data collected during a clinical trial. Its primary focus is on the accuracy and quality of this data.
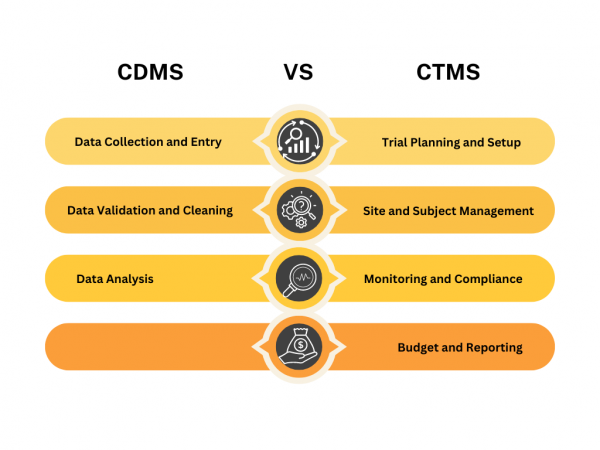
- Data Collection and Entry: CDMS helps in designing and managing the data collection forms, like electronic case report forms (eCRFs), that capture trial data.
- Data Validation and Cleaning: It ensures that the data entered is correct and complete, identifying and correcting any errors or inconsistencies.
- Data Analysis: After validation, CDMS aids in analyzing the data to help researchers make informed decisions and prepare reports.
The goal of a CDMS is to provide a reliable and accurate dataset that supports the study’s objectives and regulatory submissions.
What is a Clinical Trial Management System (CTMS)?
Clinical Trial Management System (CTMS) is a comprehensive tool designed to oversee and manage the entire lifecycle of a clinical trial. It goes beyond data management and includes various aspects of trial operations.
- Trial Planning and Setup: CTMS helps in planning the trial, setting up schedules, and managing site selections.
- Site and Subject Management: It tracks patient recruitment, site performance, and participant management.
- Monitoring and Compliance: CTMS monitors the trial’s progress, ensures compliance with regulations, and manages trial documentation.
- Budget and Reporting: It handles budgeting, financial tracking, and generates reports on trial status and performance.
The goal of a CTMS is to streamline and coordinate the overall trial process, improving efficiency and ensuring that all aspects of the trial are managed effectively.
The Main Differences Between CDMS and CTMS
Focus
CDMS is primarily concerned with managing and ensuring the quality of clinical trial data. CTMS, however, handles the broader scope of trial management, including planning, coordination, and compliance.
Functionality
CDMS deals with data-related tasks such as data collection, validation, and analysis. CTMS covers a wider range of activities, including site management, trial planning, and tracking.
Usage
CDMS is used by data managers and clinical data teams to ensure data accuracy. CTMS is used by trial managers, coordinators, and other stakeholders involved in overseeing the entire trial process.
Integration
While CDMS focuses on data, it often integrates with CTMS to provide a comprehensive view of both data and trial management aspects.



