Blog Flex Databases
As we grow not only by numbers but also by the number of different nationalities in our company, we focus more on our internal cross-cultural communication processes. Sometimes we are all very different, and it is true, but we are all the same when it comes to delivering the best result and fostering eco-communication at […]
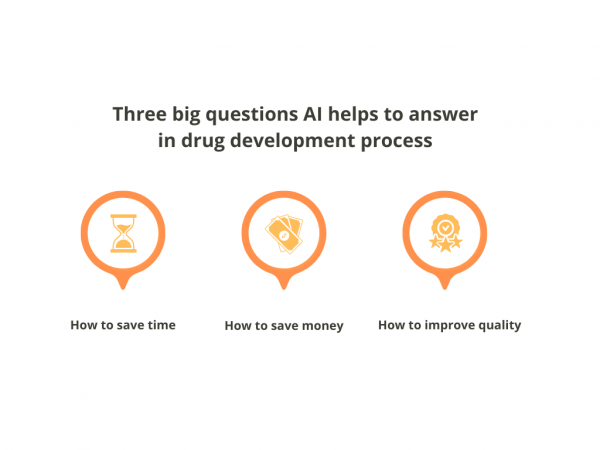
There are three big questions in any industry, including pharmaceuticals & clinical trials: Artificial intelligence is one of the new answers to all of them. Here are a few examples: When it comes to saving time, AI is here to take over data processing – fishing for answers to most minor questions in huge data […]
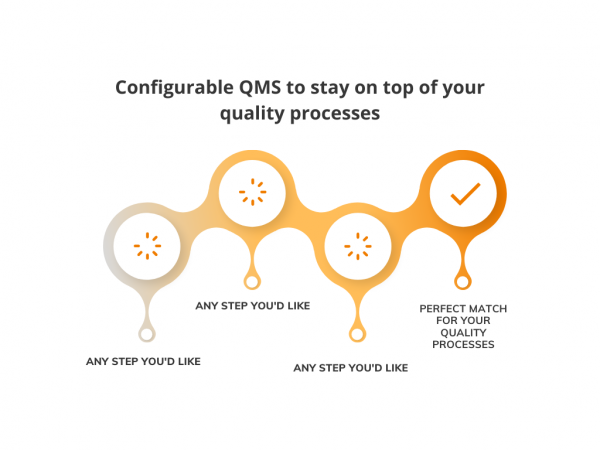
Implementing Quality Management System might be a struggle. You have it perfect on paper, but you must comply with the harsh reality of your software vendor’s system logic. Well, it’s not a problem anymore with Flex Databases QMS. In our most recent update, we’ve made every process within QMS fully configurable. You get the LEGO blocks […]
We’re excited to announce a new client – a US-based biotech iRenix Medical. Earlier this year, iRenix team selected Flex Databases as an eTMF provider. We’ve asked Stephen J. Smith, MD, Founder and CEO at iRenix Medical a couple of questions about the upcoming partnership: Why iRenix Medical decided to implement eTMF module? What was the […]
We’re excited to kick-off events of 2023 by visiting SCOPE 2023 in Orlando, the US. To meet with our one and only Business Development Director Evgenia Michalčuk-Vráblík and Product Director Vlad Kolbatov, simply step by our booth #728. Can’t wait to meet you all!
We’re starting our 2023 with some great news about a new client – Belgian Anima Research Center selected Flex Databases as a LMS & QMS provider. We have asked Linde Buntinx, Chief Development Officer at Anima, a couple of questions about the upcoming partnership: Anima Research Center is continuously innovating and optimizing their clinical study […]
Flex Databases launches Innovation Unit within Teknokent Antalya to further develop advanced solution for data-informed decisions in drug development. We’ve ended 2022 on a high note – by being accepted into Teknokent Antalya as residents with our Innovation Unit. Flex Databases Innovation Unit aims to further develop our Powerful Datahouse to address the needs of […]
Software selection and assessment is a complicated and sometimes confusing process, especially if you have to pick a provider in a hurry or if it’s your first time doing so. We’ve gathered a short list of mandatory criteria for you to check out – and have an excellent start to your EDC selection journey. Investigators […]
Clinergy Health Research recently selected Flex Databases as Pharmacovigilance software provider. We’ve asked Suyh Lin, Clinical Project Manager at Clinergy Health Research a couple of questions about the upcoming partnership: PV system and digitalization was implemented due to one of our client’s needs, and considering the expectation of an important number of safety cases to […]
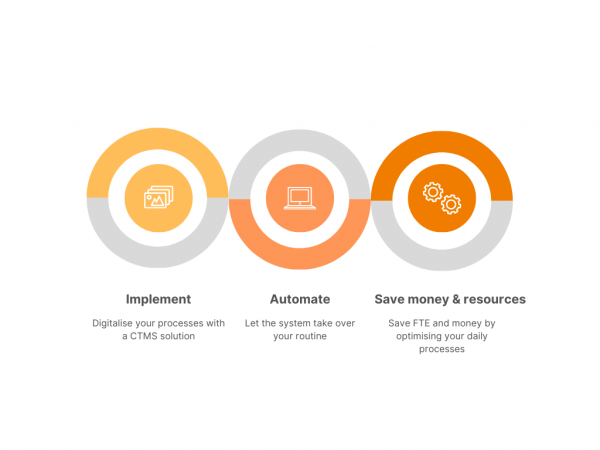
Clinical trials, as everything else in the world, is undergoing a heavy shift to process digitalization and automation. It has its ups and downs, but the future looks bright – we can minimize human error, optimize, and automate our processes and save money. Today we’ll talk about how exactly you can move from spending money […]
We kept six main questions in mind during the development of milestones. Do you want to know the answers? Milestones help us to: Plus, milestones demonstrate the trial’s success and completion in achieving financial targets. When you create a project in the system and enter its name, you can add the milestones. Doing that allows […]
November 17th, 2022 at 17.00 CET we’ll be hosting a webinar about actionable milestones that you can use to connect & control project performance throughout the Flex Databases system, including CTMS & eTMF. Connected milestones simplify control & assessment of project performance and provide you with a full transparent picture of it. Webinar host: Mountacer Benyahya, Customer […]