Blog Flex Databases
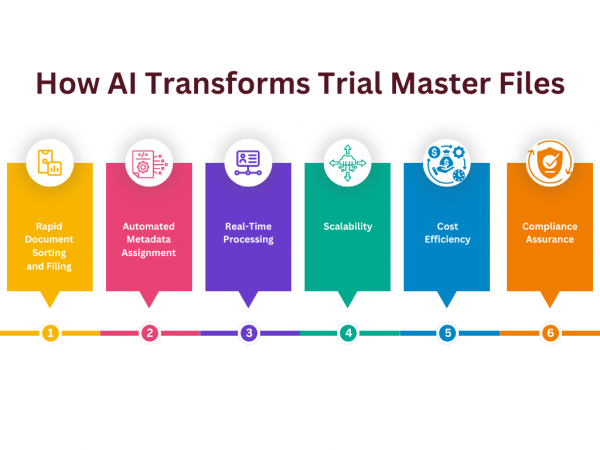
The Trial Master File (TMF) is the cornerstone of clinical trial documentation, serving as the definitive record of compliance with regulatory standards and ethical guidelines. Yet, the management of TMFs has grown increasingly complex with the rise in document volume, diversity, and stringent compliance requirements. Artificial intelligence (AI) is poised to address these challenges, introducing […]
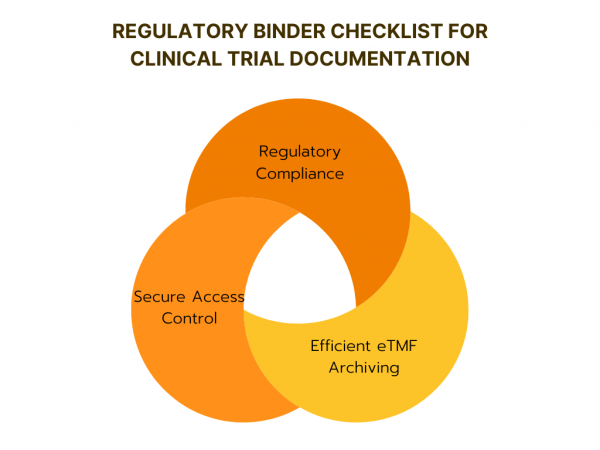
A regulatory binder is crucial for managing clinical trial documents, it ensures compliance with regulations, and makes audits easier. It helps keep important documents organized and readily accessible for trial monitors, auditors, and regulatory authorities, and serves as a useful reference for the research team. While it’s not legally required, maintaining a regulatory binder is […]
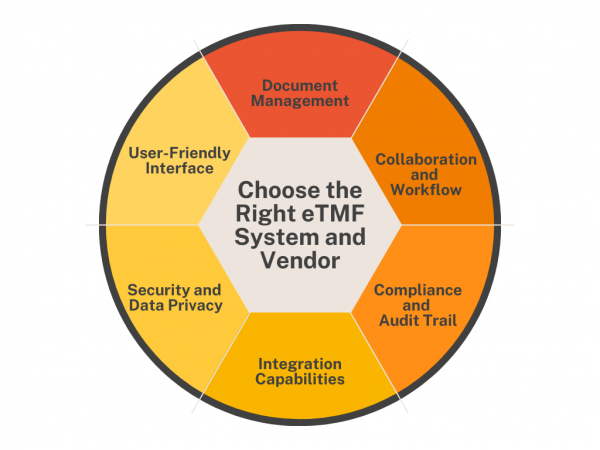
Trial master files (TMFs) are essential for completing clinical studies, but managing TMF content can be very complex. Electronic trial master file (eTMF) systems represent the next step in clinical trial document management and are becoming an integral part of future clinical research. What is an eTMF? An electronic trial master file (eTMF) is a […]
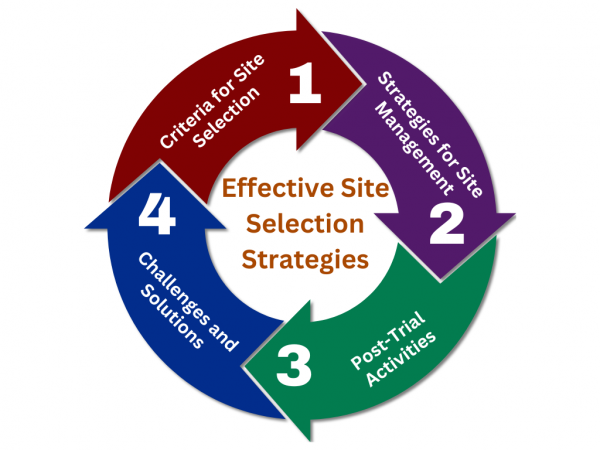
Effective site selection and management are critical components of successful clinical trials. Choosing the right locations and overseeing their operations can significantly impact the trial’s timeline, budget, and ultimately, its success in delivering reliable data. This article explores essential strategies for optimizing site selection and management, highlighting key considerations, practical tips, and the role of […]
Handling a Trial Master File (TMF) can be tough for clinical teams, but it doesn’t have to be. Old systems with outdated file sharing methods or homemade systems with spreadsheets or paper can make managing sensitive trial data risky. Over the past decade, electronic Trial Master File (eTMF) systems have helped many biopharma companies and […]
In today’s busy world of clinical research, managing documents efficiently is crucial. Imagine being able to organize hundreds of documents in just minutes. Here’s how Flex Databases’ AI-powered Trial Master File (TMF) system makes this possible. From Chaos to Order in a Few Clicks The process is remarkably simple. Users can drag and drop a […]
While AI might sound futuristic, its role in document management is simply the next step in automation. Imagine a system that automatically files the documents and assigns relevant metadata. This is now possible with Flex Databases’ AI-powered solution. But before we delve deeper, let’s address a key point: AI is not a magic bullet. It’s […]
What is an eTMF? An eTMF, or electronic Trial Master File, is a secure system used to manage all the essential documents and data for a clinical trial. It acts like a central library for everything related to the trial, from study protocols to investigator brochures. eTMF helps ensure everything is organized, easy to find, […]
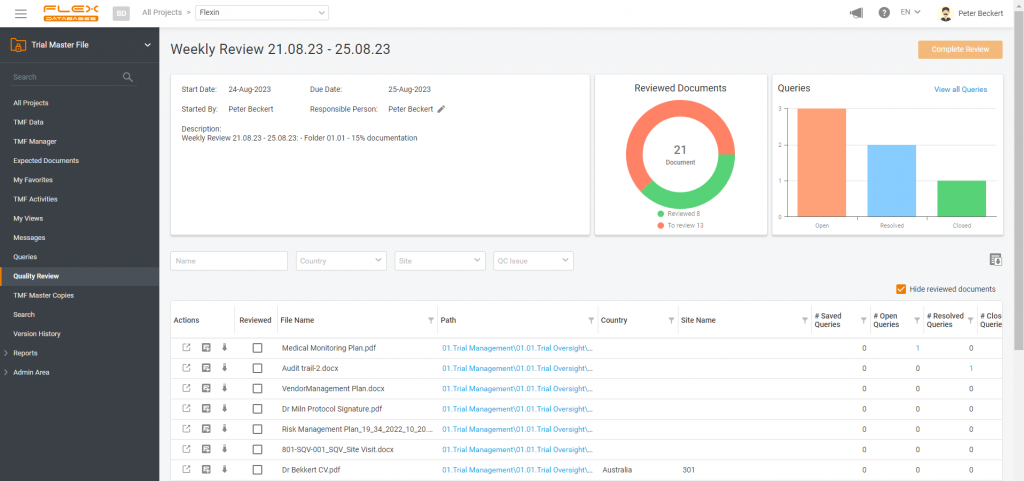
Periodic TMF reviews automatically documented. Quality and completeness under control! Let’s address an intriguing issue in the clinical trial landscape. It appears that while one third of sponsors are less than satisfied with the quality of clinical trials, an overwhelming 90% of CROs are confident in their high-quality delivery. The solution might lie in implementing […]
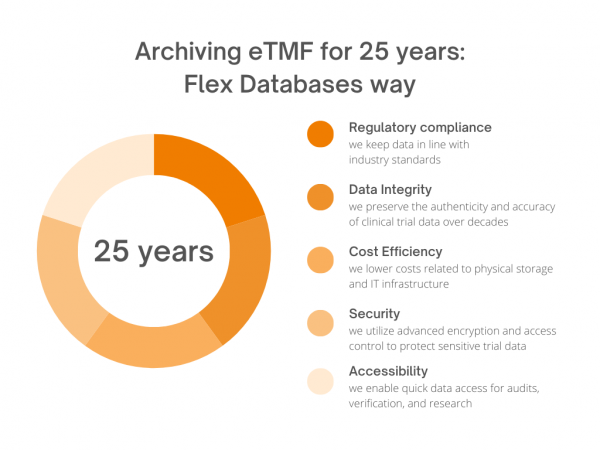
Good Clinical Practice guidelines require clinical trial documents to be kept for at least two years after the last marketing application approval in an ICH region. These documents may need to be stored longer if regulations or the sponsor require it. The EU Clinical Trial Regulation (CTR) 536/2014 demands that the content of the clinical […]
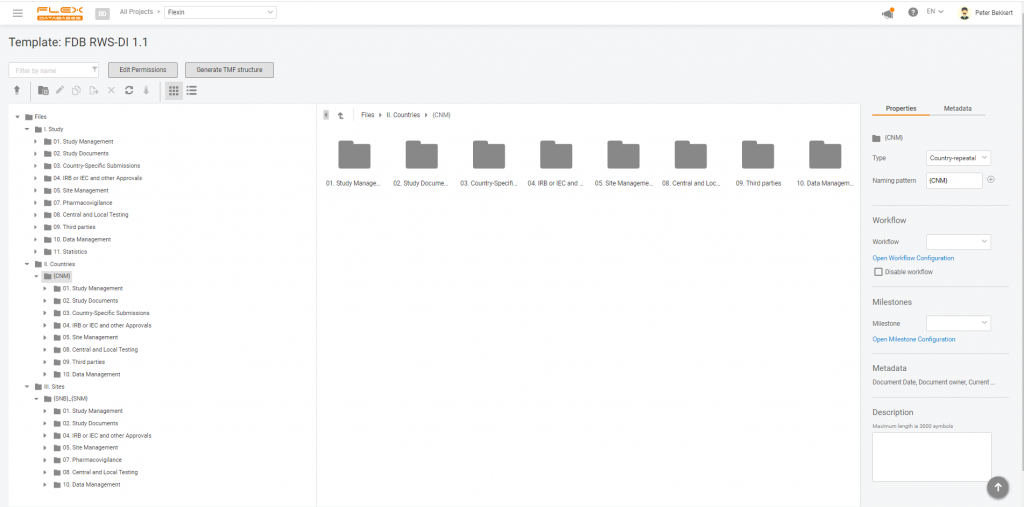
As we all know, the eTMF structure for RWE studies differs from a general TMF Reference Model. Here’s the list of main differences: To make everyone’s lives easier, we added a template for RWS to our eTMF – you can select it when you create an eTMF structure for the project from the list of […]
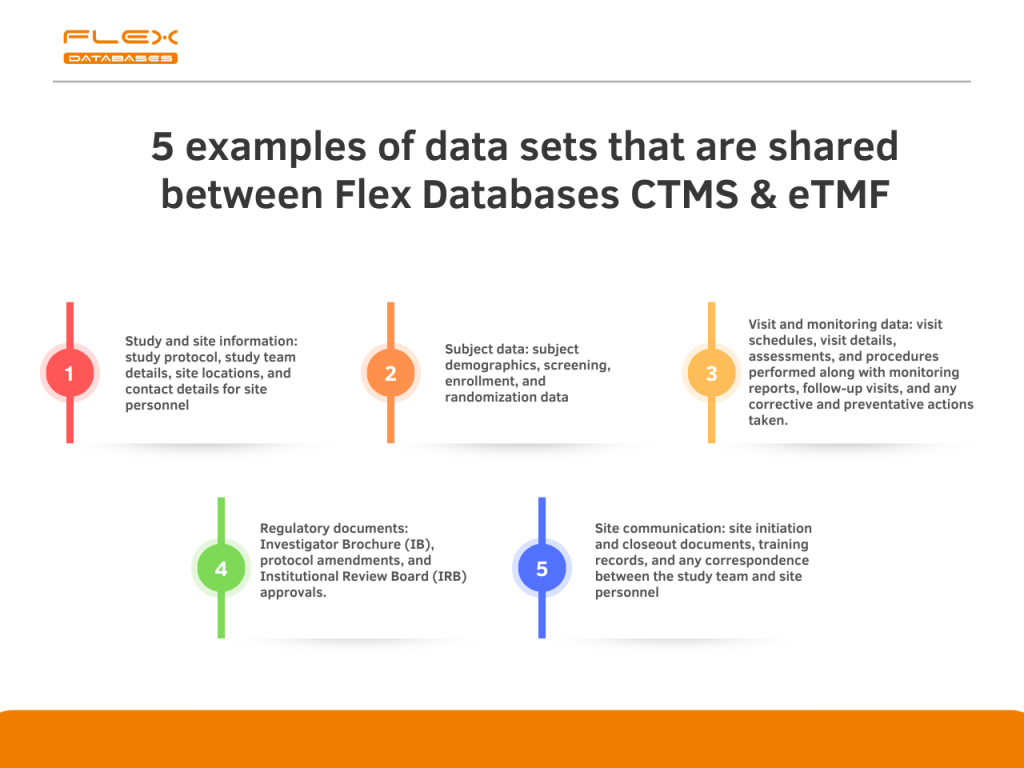
A study published in the Journal of Medical Systems found that integrating eTMF and CTMS systems resulted in a 50% reduction in the time required to identify missing documents and a 75% reduction in the time required to resolve missing document issues. Flex Databases CTMS & eTMF are interconnected to deliver you the highest possible […]