Blog Flex Databases
Flex Databases Solves One of the Biggest Document Management Headaches Clinical teams have long struggled with one persistent problem: managing TMF documents across multiple systems, versions, and stakeholders. Switching between platforms, downloading files, losing track of versions, and waiting for colleagues to finish editing slows down operations and increases compliance risks. With this update, Flex […]
📂 Last-minute TMF quality reviews cause stress, missed documents, and higher audit risks.With Flex Databases, you avoid the scramble – reviews are structured, transparent, and always under your control. With Flex Databases, you get:✅ Control over the process✅ Full transparency and traceability✅ Less stress before audits✅ Clear outcomes in one report 🎥 See how it […]
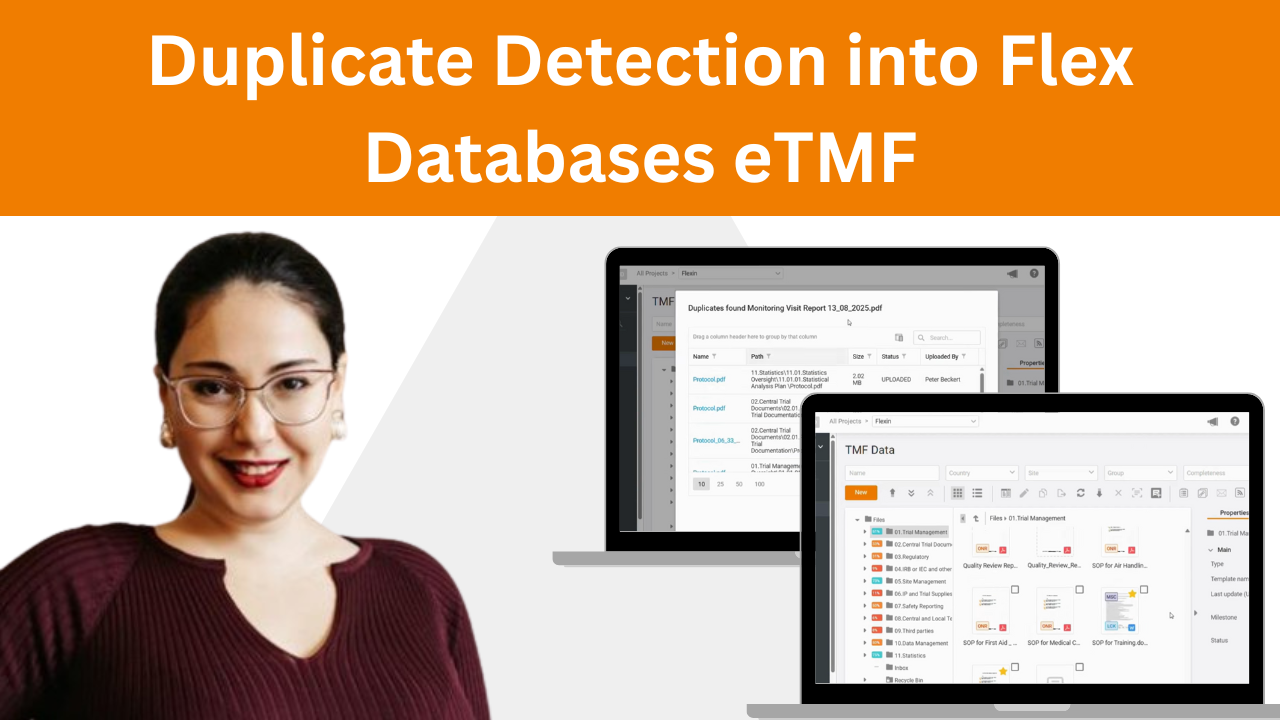
Ever uploaded a document to your Trial Master File only to realize it was already there?You’re not alone! Duplicates waste time, clutter your TMF, and create compliance risks. That’s why we built duplicate detection into Flex Databases eTMF. ✅ Real-time scanning as soon as you upload✅ Checks both file name and document content✅ Skip, replace, […]
Ever uploaded a document to your Trial Master File only to realize it was already there?You’re not alone! Duplicates waste time, clutter your TMF, and create compliance risks. That’s why we built duplicate detection into Flex Databases eTMF. ✅ Real-time scanning as soon as you upload✅ Checks both file name and document content✅ Skip, replace, […]
When preparing a tender for a Clinical Trial Management System (CTMS) and an electronic Trial Master File (eTMF), CROs must choose solutions that meet sponsor expectations and improve operational efficiency while keeping studies inspection-ready. The right choice affects study delivery, compliance, and client satisfaction. Below are the key factors CROs should consider. Regulatory Compliance and […]
A Comparative Look at Europe vs. US Markets In clinical operations, time is money – but how much money can technology really save you? We analyzed the return on investment (ROI) for implementing electronic Trial Master File (eTMF) and Clinical Trial Management Systems (CTMS) based on real-world benchmarks. By modeling savings from reduced manual effort, […]
Many clinical trial teams still rely on a legacy TMF – whether that means physical binders or outdated digital folders lacking structure and traceability. These systems often fall short in today’s increasingly regulated, fast-paced environment, making oversight and compliance more difficult. That’s why more sponsors and CROs are transitioning to a modern eTMF. A modern […]
An electronic Trial Master File plays a central role in maintaining oversight, ensuring regulatory compliance, and supporting successful inspections by health authorities. An efficient eTMF is no longer optional – it’s essential. Choosing the right eTMF model for your clinical studies directly impacts how effectively your teams can manage documentation, maintain version control, and collaborate […]
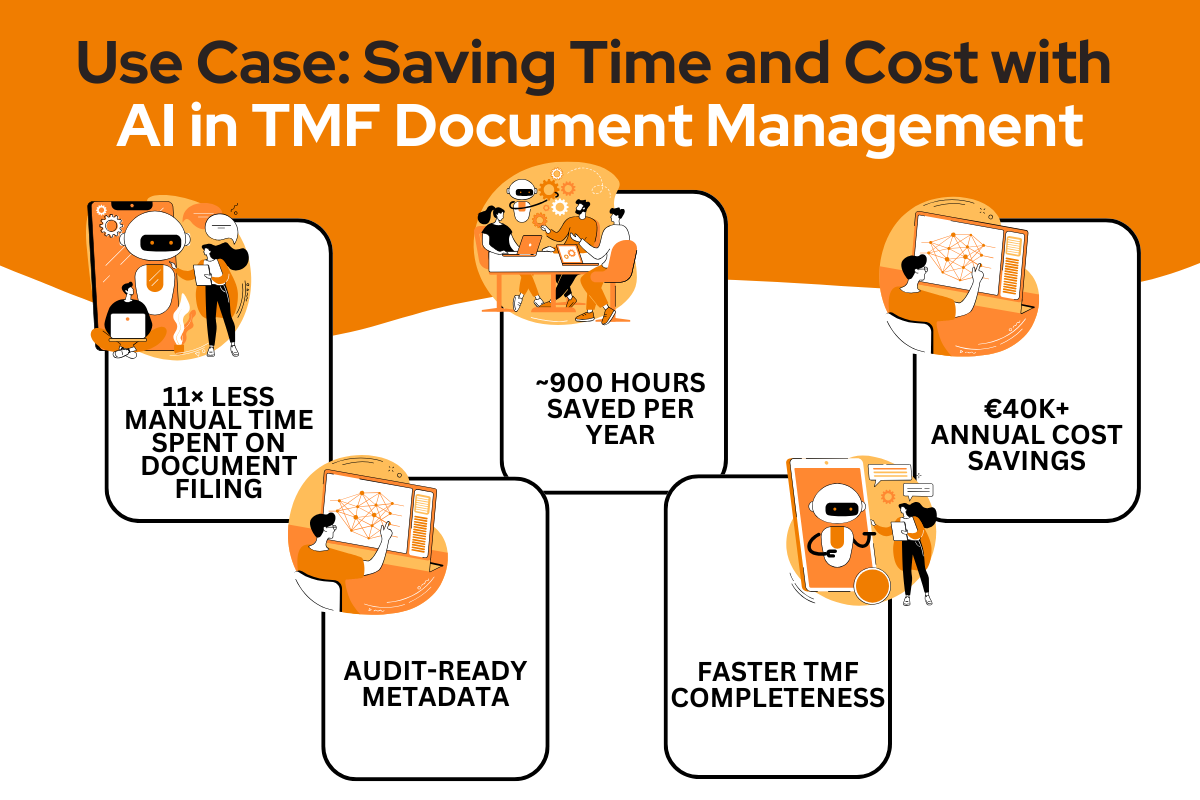
A mid-sized CRO managing 9,500 TMF documents annually faced increasing challenges with filing and metadata assignment. Each document required an average of 5–6 minutes to process manually. The Problem Manual document upload and metadata entry amounted to: This process also posed risks of human error and inconsistency. The Solution The CRO implemented an AI-powered eTMF […]
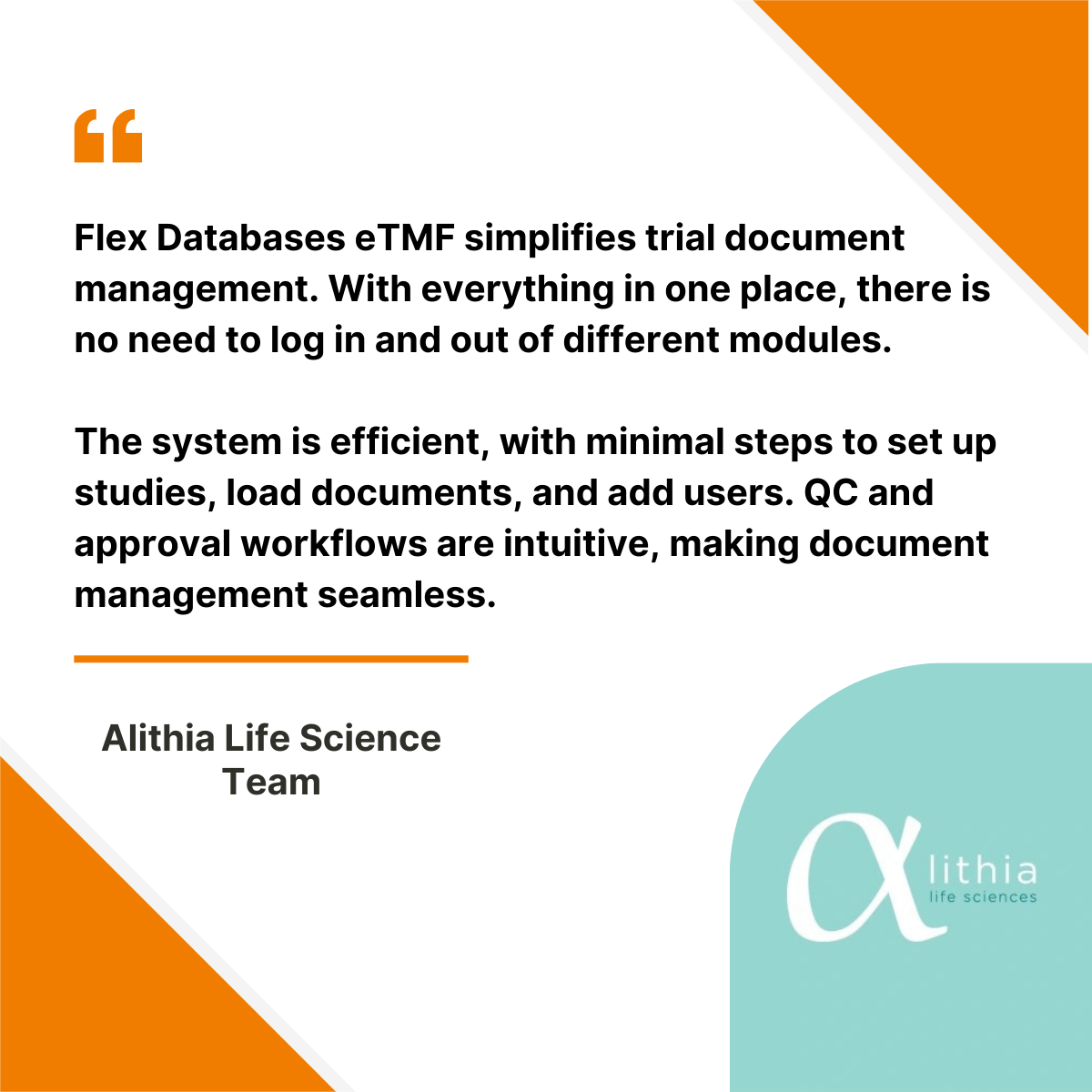
Alithia Life Sciences is a full-service Contract Research Organization dedicated to deliver high-quality clinical trial management solutions. With a strong focus on efficiency, compliance, and operational excellence, Alithia partners with pharmaceutical and biotech companies to ensure the seamless execution of clinical trials. To further enhance its document management and trial oversight, the company selected Flex […]
At Flex Databases, we are committed to delivering cutting-edge solutions that streamline clinical trial management. Our latest updates bring powerful enhancements to the Investigators & Sites Management module and Trial Master File, helping users gain better insights, improve workflows, and ensure seamless compliance. Investigators & Sites Management: Stay Ahead with a Powerful Dashboard Our newly […]
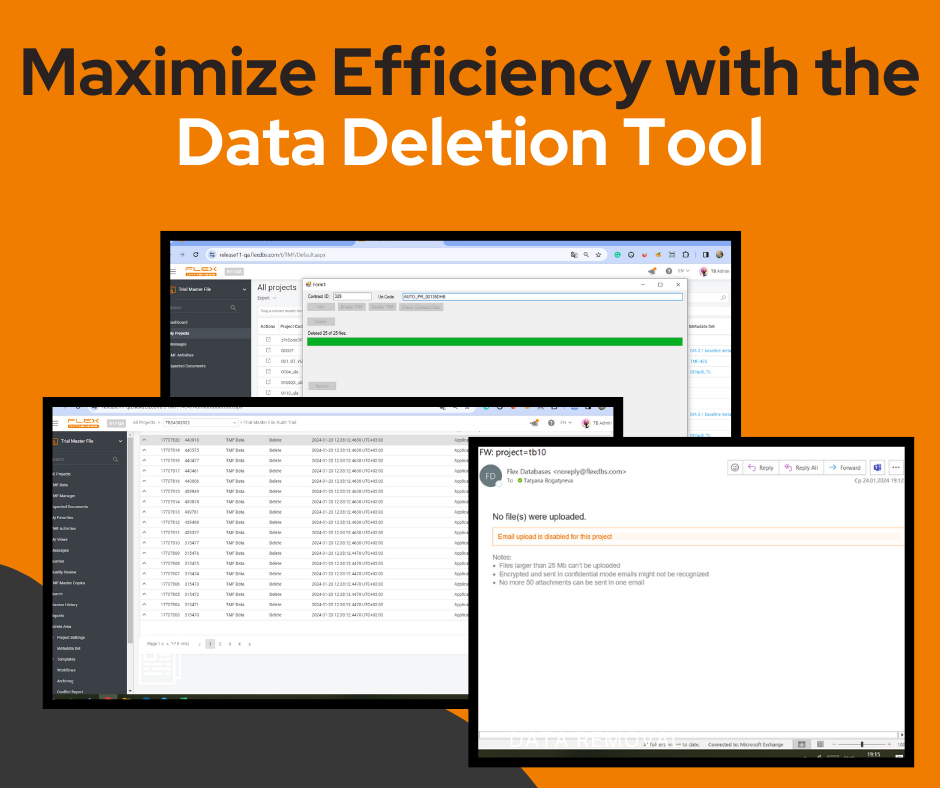
The Data Deletion Application is an advanced tool that helps organizations manage storage, meet regulations, and improve data security. It allows secure removal of entire studies from Trial Master File storage, giving businesses full control over sensitive information. Key Benefits Seamless & Secure Data Management Comprehensive Data Removal The application permanently deletes: Complete Transparency & […]