Blog Flex Databases
Cross-site budget setup can turn into hours of manual per-subject cost entry, slowing teams down and creating error risks. With Flex Databases’ Subject Tracking & Invoicing, you can now import Per Subject Costs directly from Excel using the Data Import Wizard. ✔ Speed up site budget setup✔ Reduce manual entry and errors✔ Import across projects […]
In July 2025, the European Commission adopted Commission Implementing Regulation (EU) 2025/1466, amending Regulation (EU) No 520/2012. The changes aim to strengthen pharmacovigilance, reduce unnecessary administrative burden, and align EU requirements with global best practices. Most provisions apply from 12 February 2026, with certain Eudravigilance updates taking effect in August 2025. Why the Changes Were […]
Palobiofarma is a leading clinical-stage biotech company dedicated to developing innovative medicines. Our focus on adenosine signaling drives our commitment to scientific excellence, with six drug candidates currently in different phases of development. Among them, PBF-999 for Prader-Willi Syndrome stands out as a key project. Our mission is to advance patient care by leveraging cutting-edge […]
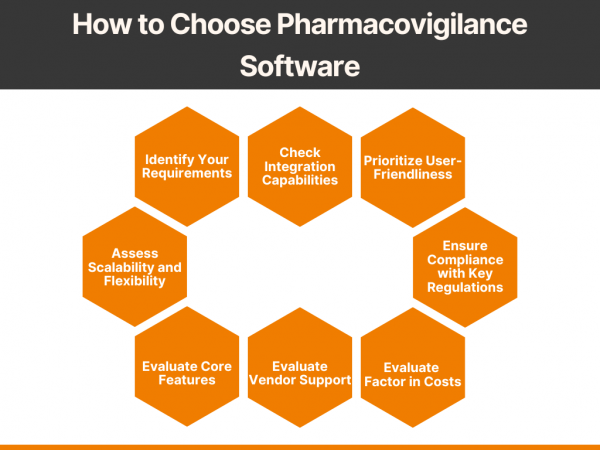
Pharmacovigilance (PV) is a critical aspect of drug safety, focusing on detecting, assessing, understanding, and preventing adverse drug reactions or other drug-related problems. As global regulatory requirements for drug safety evolve, adopting reliable pharmacovigilance software has become essential for ensuring compliance and improving operational efficiency. Selecting the right PV software can significantly impact your organization’s […]
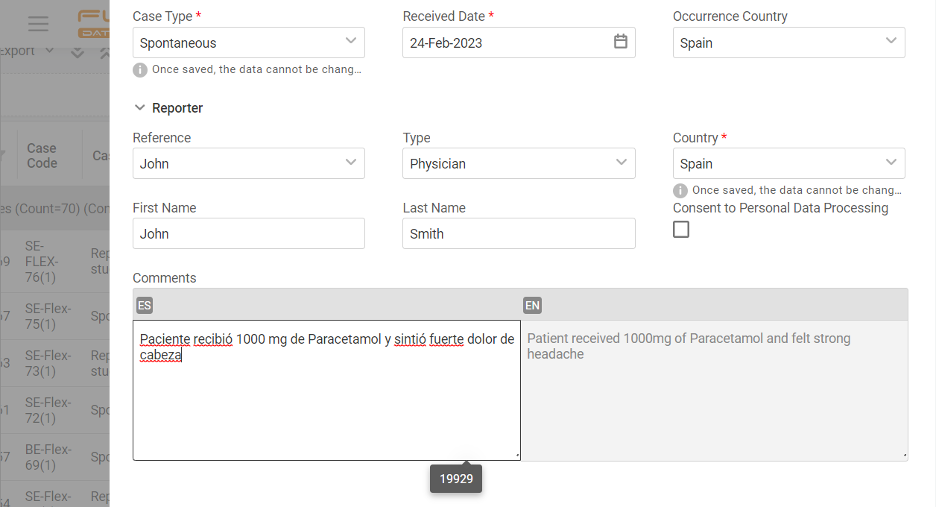
If you operate globally, you must comply with local language requirements, which also apply to pharmacovigilance reporting. Imagine that you must manage cases & report in English, Spanish, and French. How would you do that? The apparent answer many providers usually offer is multiple inputs of the same cases. Drop that! With Flex Databases Pharmacovigilance, […]
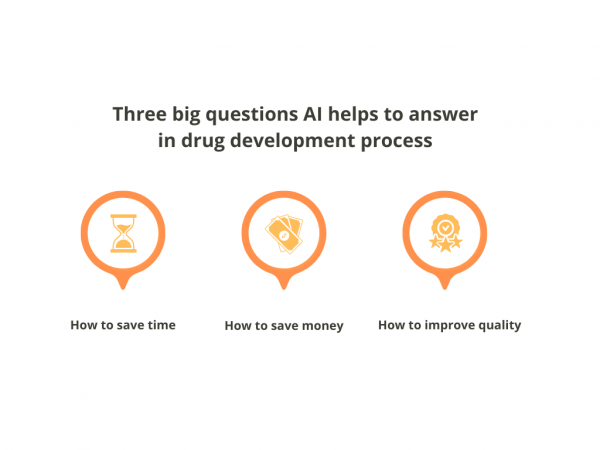
There are three big questions in any industry, including pharmaceuticals & clinical trials: Artificial intelligence is one of the new answers to all of them. Here are a few examples: When it comes to saving time, AI is here to take over data processing – fishing for answers to most minor questions in huge data […]
This September, Pharma Tech Outlook included Flex Databases in Top-10 Pharmacovigilance solutions providers in the UK & Europe. Our Business Development & Marketing Director Evgenia Mikhalchuk took part in a cover story for Pharma Tech Outlook September’22 issue, supporting our nomination: Clinical trials have always been about data. But, prior to automation, data input was […]
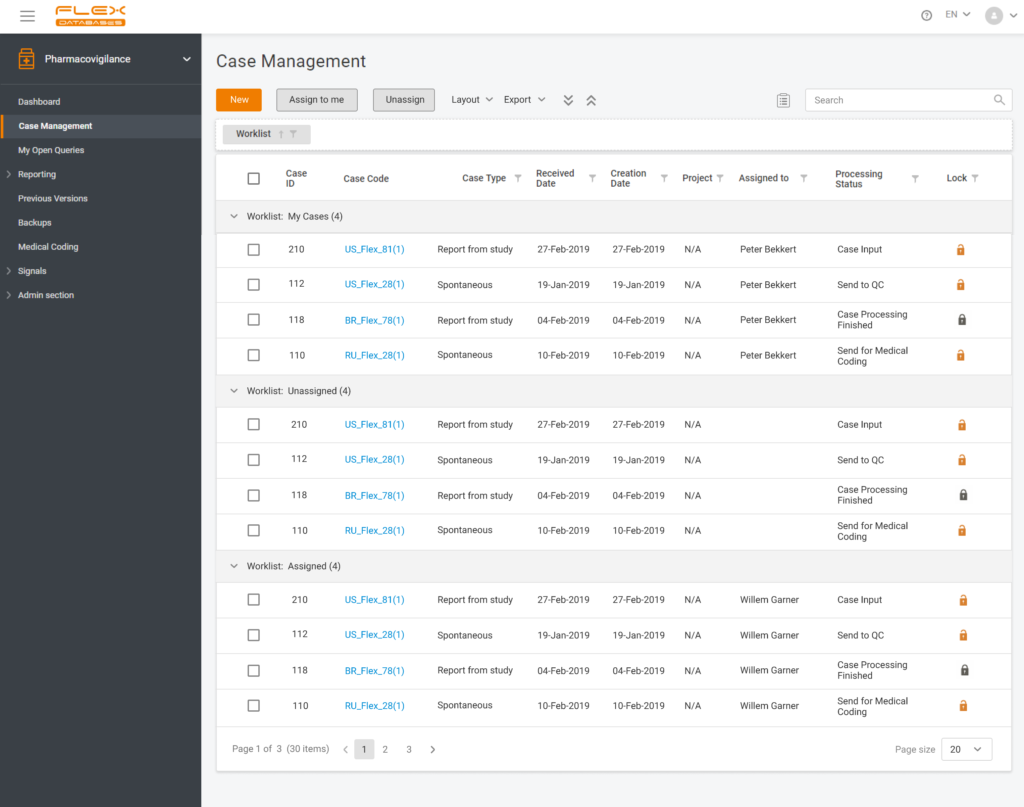
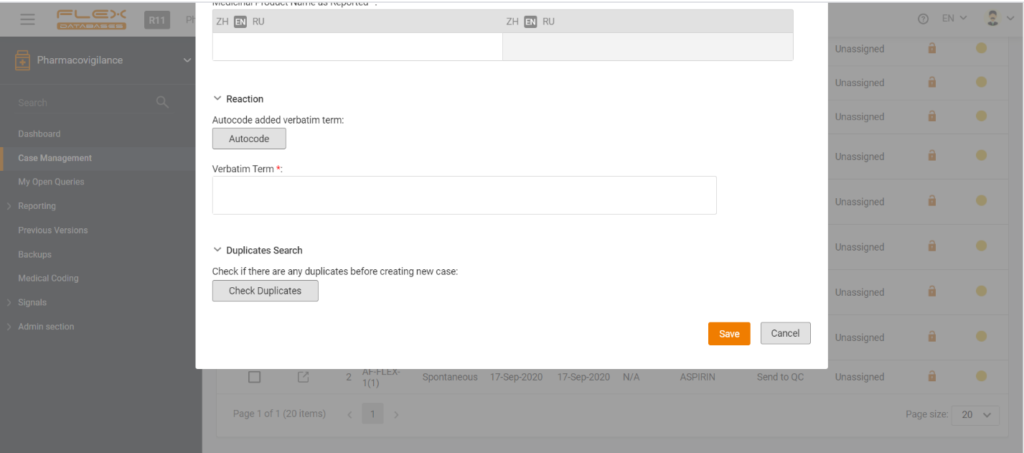
Each step of Flex Databases is always done with one thought in mind – to save time and money for our clients. That’s why to save your time and give you a deeper understanding of how our Pharmacovigilance module work, we are happy to answer the six most frequently asked questions about the system. How […]
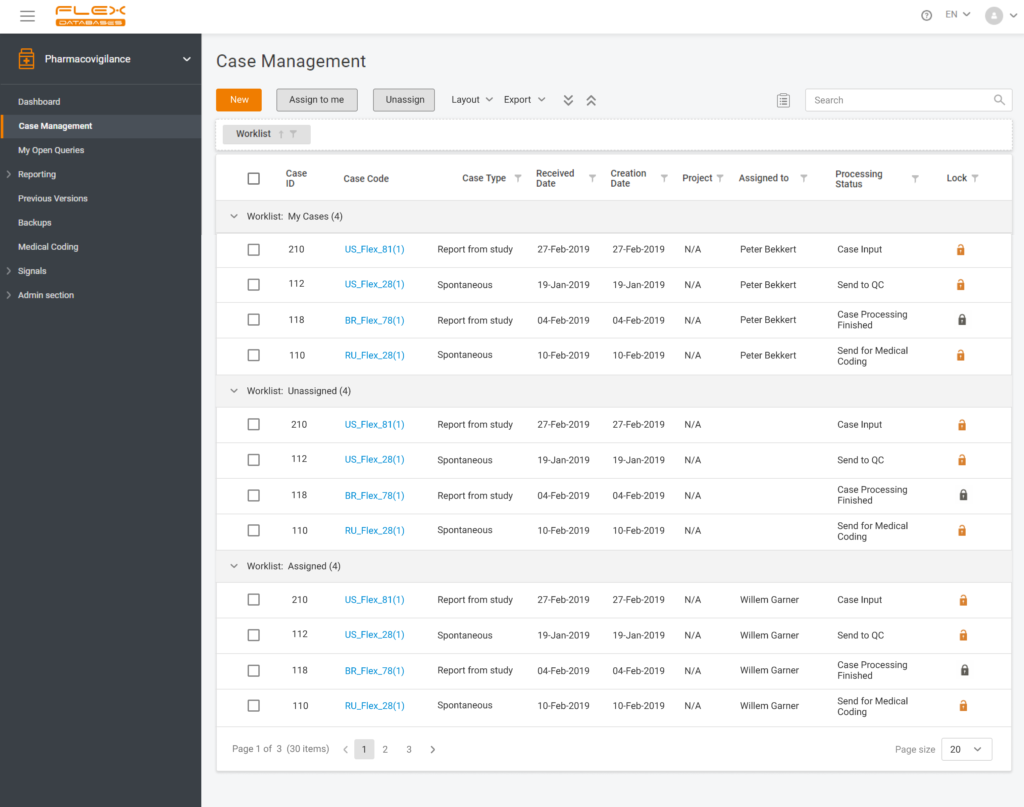
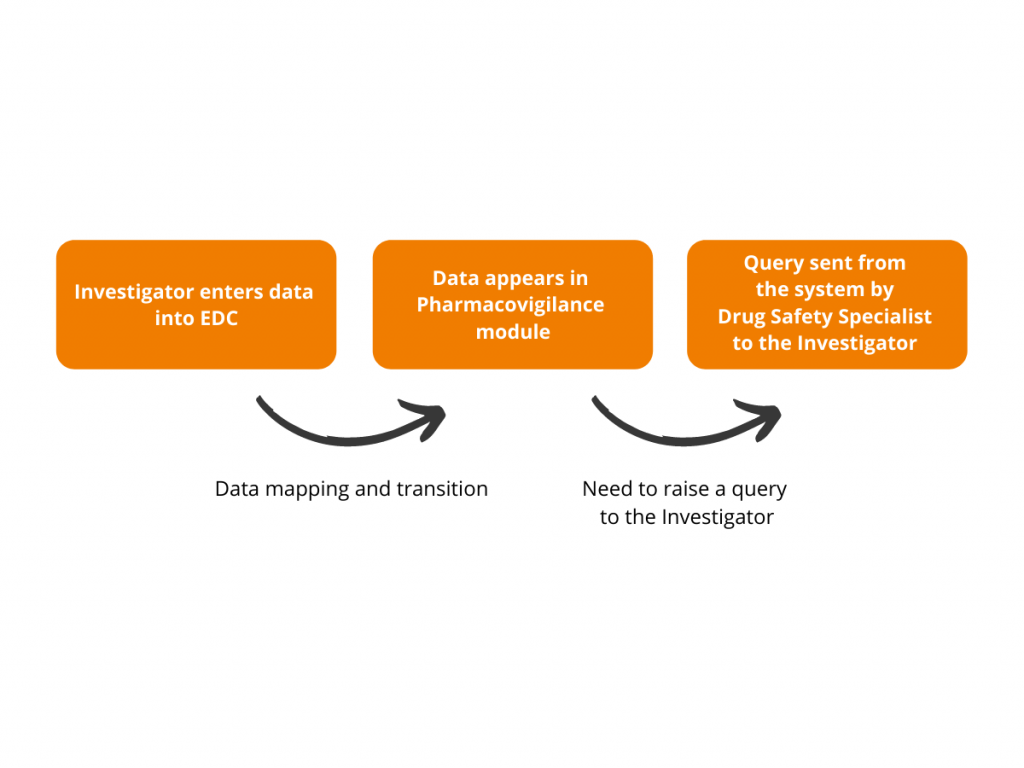
Many years since the appearance of different platforms and systems for clinical trial operations, there has been a question about making all the systems work synchronized. For most parts of the platforms and systems, it occasionally became possible, though Drug Safety Specialists still have to lead their pharmacovigilance processes independently. Having the objective to streamline […]
The development of information technology is significantly ahead of the development of regulatory and other legislation. Despite the obvious benefits of introducing new technologies in electronic systems for pharmacovigilance, it is necessary to consider the difficulties that a company will face: Validation and audits completion Personal data management Personnel training, possible changes in the organizational […]
As we briefly discussed in the previous chapter, Artificial Intelligence (AI) is used to simulate the processes of a human brain with the help of computer systems. It encompasses various technologies, including rule-following, reasoning – using rules to reach rough or specific conclusions, learning, and self-correction. Implementation areas of AI In the area of pharmacovigilance […]
At the very end of winter, Flex Databases signed a contract for CTMS and Pharmacovigilance module implementation with Italian Human & Digital CRO Exom Group. We’ve asked Luigi Visani, MD, President & CEO at Exom Group a couple of questions about the upcoming partnership: Why did Exom Group decide to pick Flex Databases as a provider? “Since its inception, […]