Flex Databases CTMS & eTMF are interconnected to deliver you the highest possible level of data completion and avoid missing documents issues.
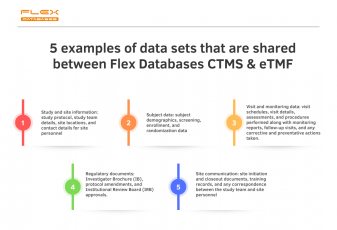

Flex Databases CTMS & eTMF are interconnected to deliver you the highest possible level of data completion and avoid missing documents issues.
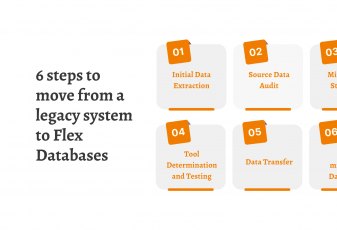
Here at Flex Databases we offer you a simple six-step approach to move all data from your legacy system to Flex, and we need your involvement only for three of those six.
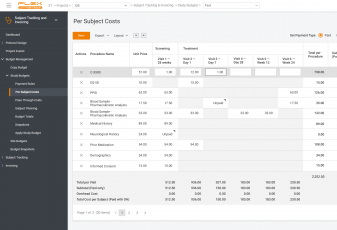
Will you get frustrated if you are paid with a half-year delay? Yeah! Will that motivate you to work more with such a partner? Doubt that.

“Why did you decide to implement CTMS?” is one of the first questions our CTMS clients get during a presale. We’ve collected top-5 answers for you to check and see if something hits close to home, and it’s time to start thinking about your own CTMS implementation.

In early April 2023 we’ve hosted a webinar about why and how you can digitalize your Quality Management System with our brilliant QA Director Anna Petrovskaya as a host.

Flex Databases compliance checklist for EMA Guideline on computerised systems and electronic data in clinical trials
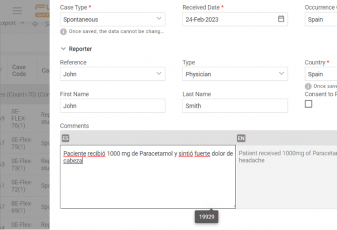
With Flex Databases Pharmacovigilance, you’ll only need one case, and you can add as many languages to it as you want.
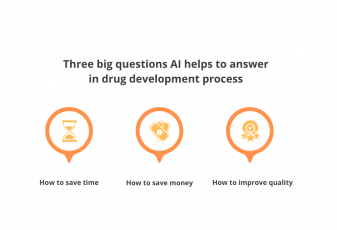
By automating processes, reducing human error, and improving decision-making, AI can help make clinical trials more efficient, effective, and safe.
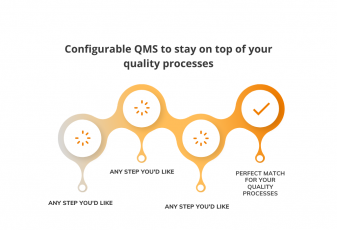
In our most recent update, we’ve made every process within QMS fully configurable. You get the LEGO blocks of different action items, a scheme being our default process workflow, and you can build it HOWEVER you want.

We’ve gathered a short list of mandatory criteria for you to check out – and have an excellent start to your EDC selection journey.
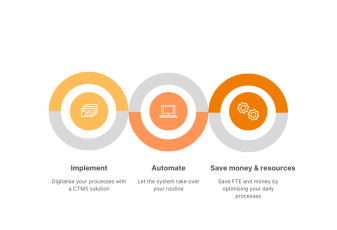
Digitalization is usually promoted as cost-effective – and here’s our take on that. Do you want to learn more?
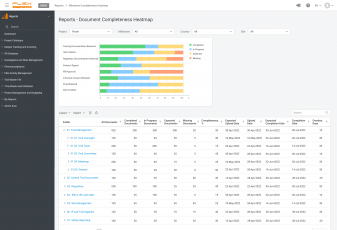
We kept six main questions in mind during the development of milestones. Do you want to know the answers?