Blog Flex Databases
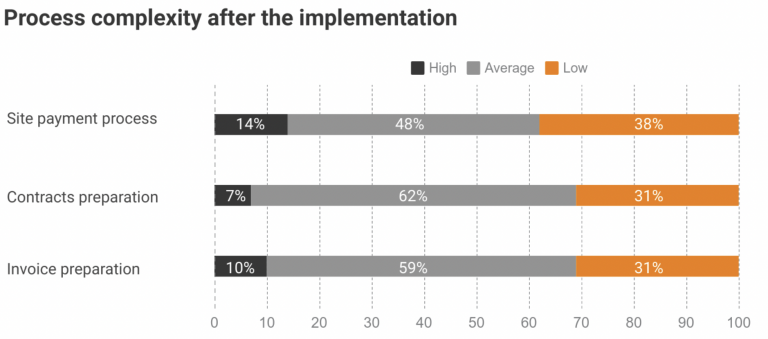
st trial sites work with no more than a 3-month operating budget. With an estimated two-thirds of trial sites falling into this category, software companies have sought to close this gap in effective financial operations with process automation and payment schedule. So CROs, pharma companies, and biotech can finally get transparency in site payments. Likewise, effective […]
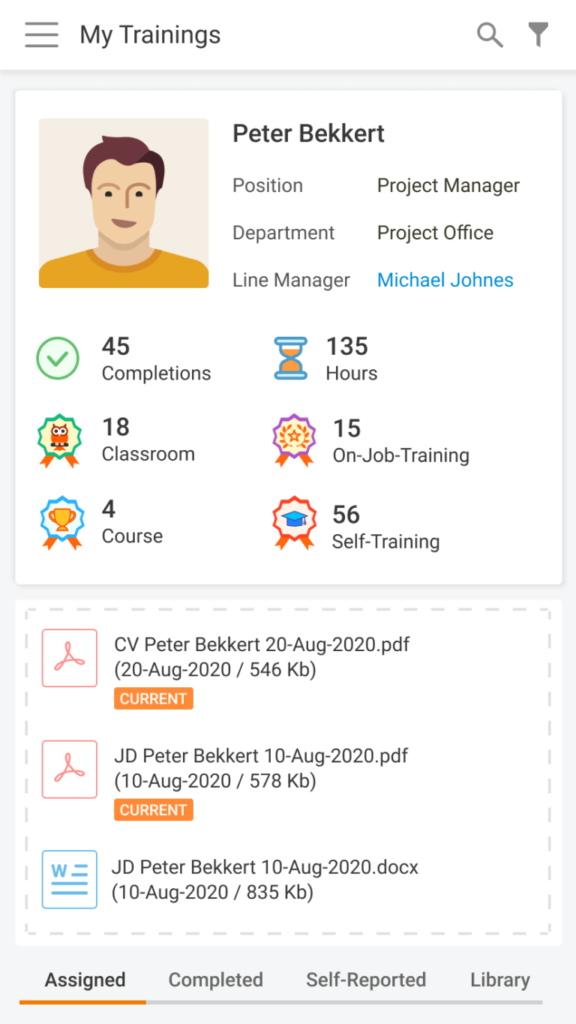
It’s been two years since our How to stop worrying and start working in clinical trials material dropped. Content-wise, it was the highest point for our Learning Management System so far. I have to admit, since then, we kind of abandoned its storytelling activities and just sometimes briefly mentioned new developments for LMS in our release notes. […]
At the end of 2020 TCD, an African-focused CRO with a full-service offering, signed a contract for eTMF implementation with Flex Databases. We’ve asked Bianca Oelschig, Clinical Data Sciences Manager/Project Manager at TCD, a couple of questions about the upcoming partnership: Why TCD decided to pick Flex Databases as a provider for eTMF? After spending many months researching […]
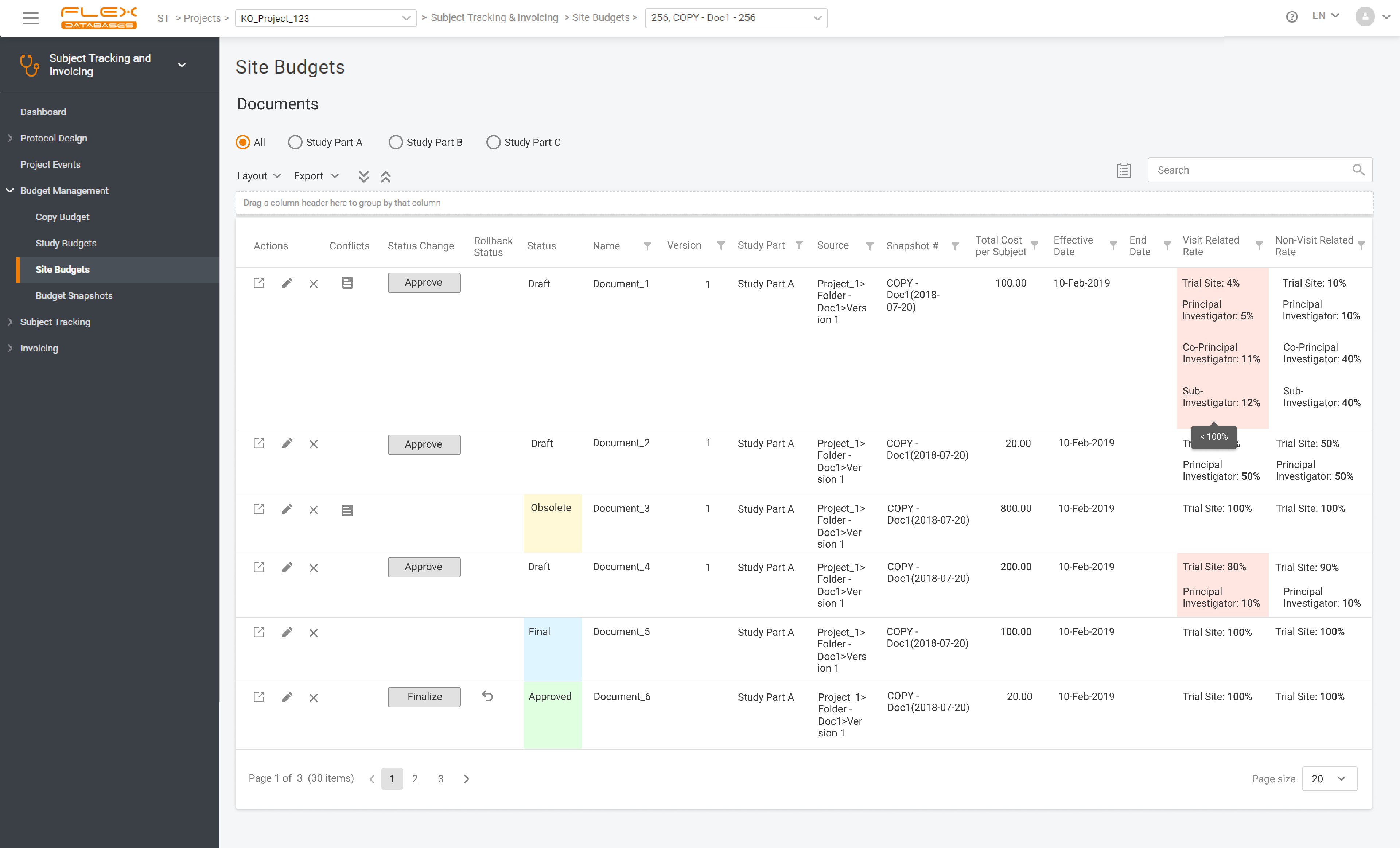
Subject Tracking & Invoicing – Flex Databases CTMS module. Use Subject Tracking & Invoicing Module to track all patients’ data and manage invoicing online. Plan and schedule patient visits efficiently. Video duration: 08:50
Thank you for being with us 2020,We wish you a happy and healthy 2021. Some of you (well, considering that it’s the 29th of December and most of us are on holidays, most of you) will read this text in 2021 and know a little bit more about a year ahead, but there’s one thing […]
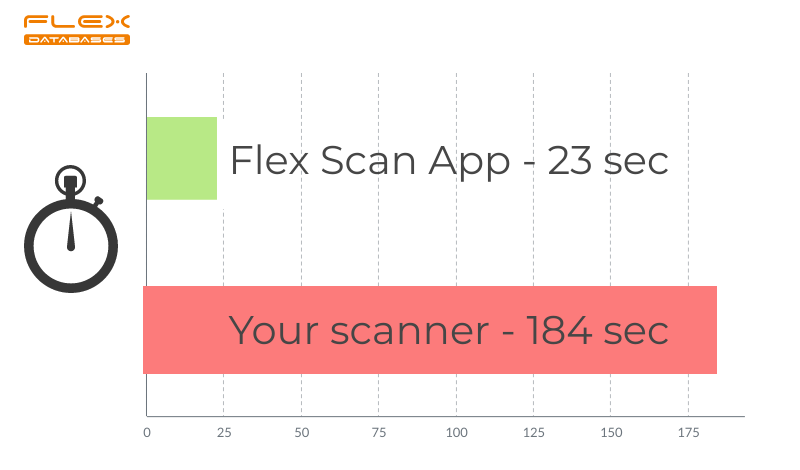
We keep making users’ lives easier! At least for early adopters. Don’t you have your mobile phone on you all the time? It’s the only thing you need to upload scanned documents into our eTMF. No more broken scanners or bad connection – just take pictures on the go and spend your time on more important tasks. […]
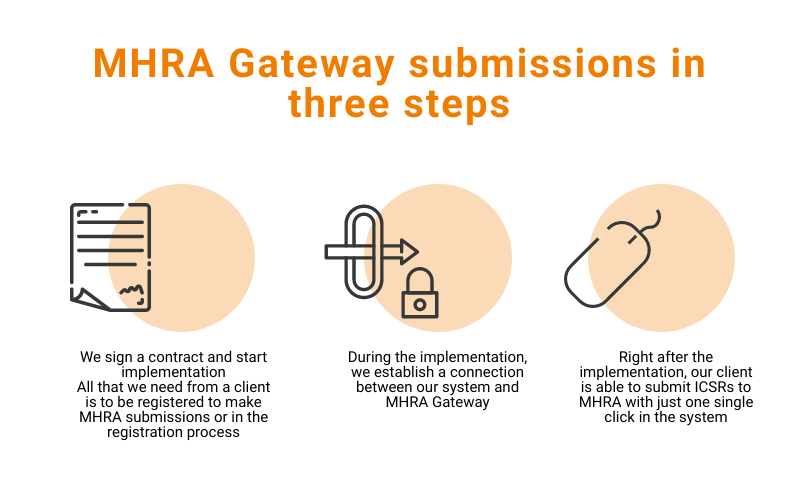
In 2021 the United Kingdom is moving to its post-Brexit life with new processes, procedures, and laws everywhere, including clinical trials and pharmacovigilance. This piece we dedicate to a simple question – how to move to post-Brexit pharmacovigilance effortlessly and efficiently. All information in this article is based on Updated guidance on pharmacovigilance procedures published by Medicines […]
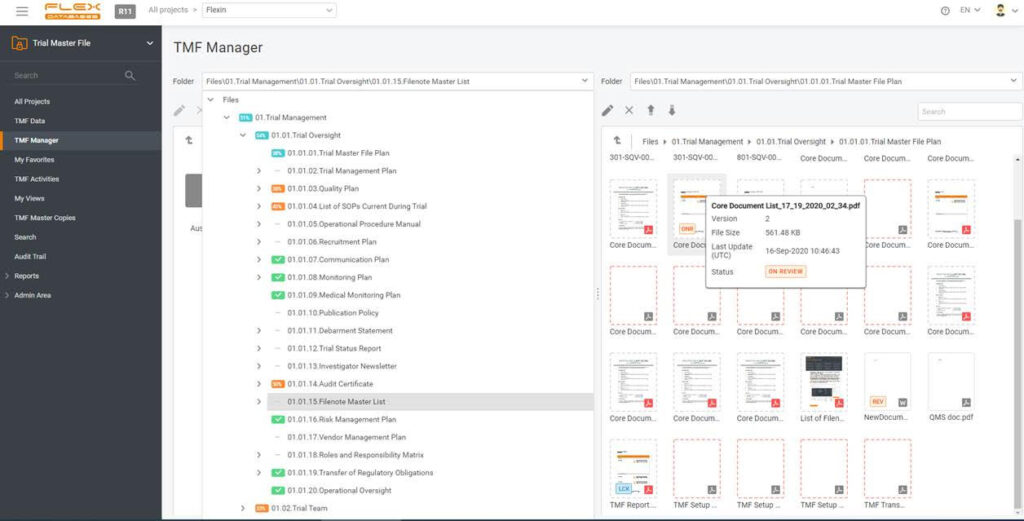
Documenting everything is a burden when running a study, especially a big and international one. Just think about putting all the documents in one place, and, most importantly, keeping this place tidy and structured. One must collect documents from many sites and can’t allow losing anything. It usually costs a fortune to train site personnel […]
When we think about project management & budgeting systems, we usually imagine something with a bad interface, no clear user logic, and an overloaded price. Well, there is something on the market that’s easy to understand and use yet still powerful, flexible, and doesn’t cost like a new jet. Since 2011 we develop and improve […]
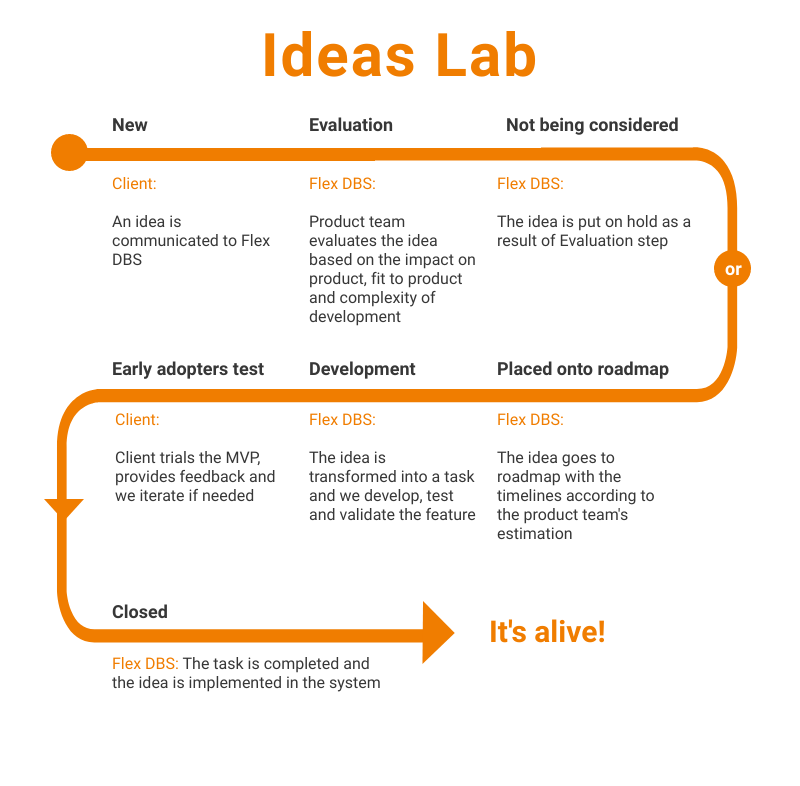
Flex Databases system is well-known for its flexibility (you get it right from our name). We continuously develop our system and add new functionality. To create a roadmap, we follow clients’ requests to stay flexible, customer-driven, and relevant. But where our clients’ ideas go? How do we make sure that nothing important is lost and […]
Australian data management service provider PureCDM has recently signed a contract with Flex Databases to implement our Project Management & Budgeting and Time Sheets & Utilization solutions. We’ve asked Helen Poliviou, Managing Director a couple of questions about the upcoming partnership: Why did PureCDM decide to pick Flex Databases as a Project Management & Budgeting, Time Sheets & Utilization provider? With […]
Bionical Emas is the only CRO to combine Clinical Development, Early Access Programs and Clinical Trial Supply, to deliver a unique, seamless approach supporting our clients to bring new medicines to patients faster. “Our partnership relationships are paramount to everything that we do, and we work closely with our clients to become an extended part of their team. We […]