Blog Flex Databases
There are many factors to consider when purchasing a clinical trial management software – from regulatory requirements to the sponsor’s wishes. Many of these factors could be summarized to a need. To comply with the need, you create a checklist with major and minor features and flows to implement. But, keeping in mind that every business is […]
It all started with a big bang small team of professionals aiming to build a masterpiece of a product and a company. Now we are almost a hundred, and it couldn’t have been better. 2011: From 5…. 2021: …to 70 and counting Vlad K, Product Director Karine D, Project Manager During my first year at Flex Databases, […]
This summer ABX-CRO and Flex Databases signed a contract for a CTMS & eTMF implementation. We asked Dr. Ulrike Schorr-Neufing, Director, Qualification & Training, ABX-CRO, a couple of questions about the upcoming partnership. Why ABX-CRO decided to pick Flex Databases as a provider? After an intensive selection and evaluation process comparing different service providers ABX-CRO […]
Even though electronic Trial Master File seems to be almost essential nowadays, there are still many questions on its value and benefits that it could bring to a company. We’ve decided to answer the most common and most important of them and walk you through the entire process – from Why select eTMF through Legacy data migration to Smart eTMF, which works […]
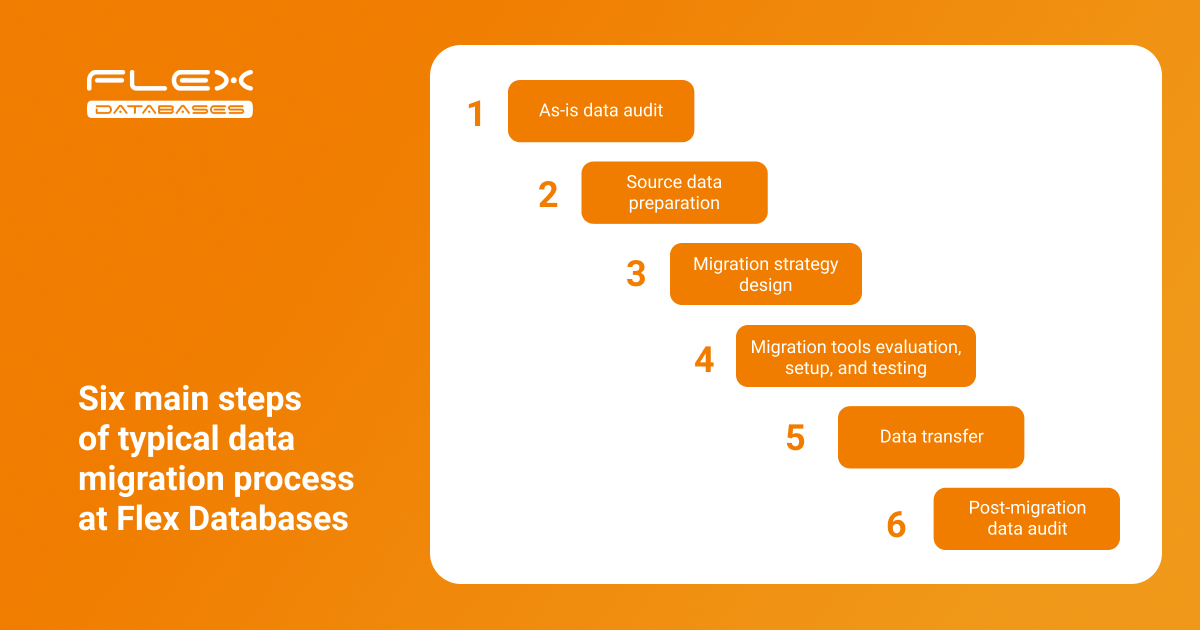
Recently, we discussed questions that might arise when changing your eClinical software provider. Today, we want to take a closer look at one of the most asked questions: How can I migrate data from a legacy CTMS or eTMF? Data migration is a delicate process with many moving parts. Without a well-established process, the CTMS go-live could […]
At the very beginning of 2021 Flex Databases and German FGK CRO signed a contract for CTMS and eTMF implementation. About FGK FGK CRO is a full service contract research organization offering a complete range of clinical development and consulting services to pharmaceutical, biotechnology and medical device companies. FGK approaches each project, whether large or small, with dedicated, highly motivated, small […]
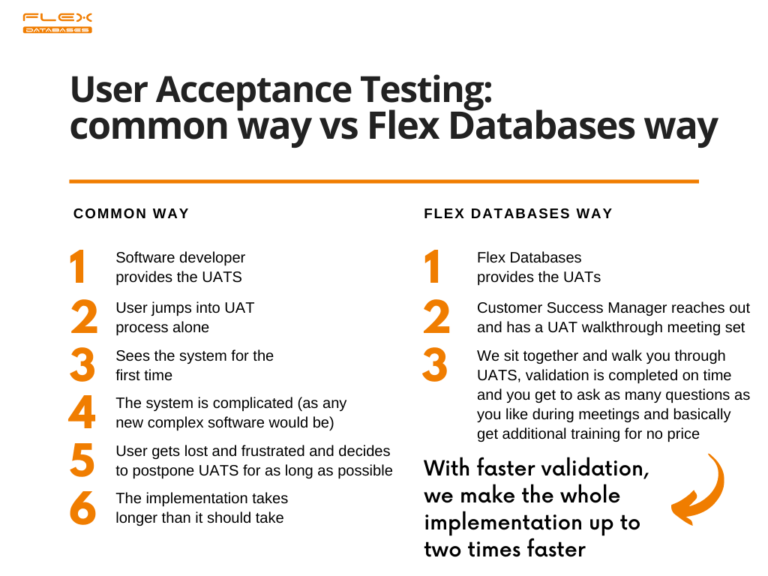
Software validation is tricky, especially for those who’s never met it before. Still, it Is required, and it can affect implementation timelines in the worst way if handled poorly. That’s why we at Flex Databases developed a simple workflow to make validation almost effortless and as simple as possible: Step 1: We provide the happy new […]
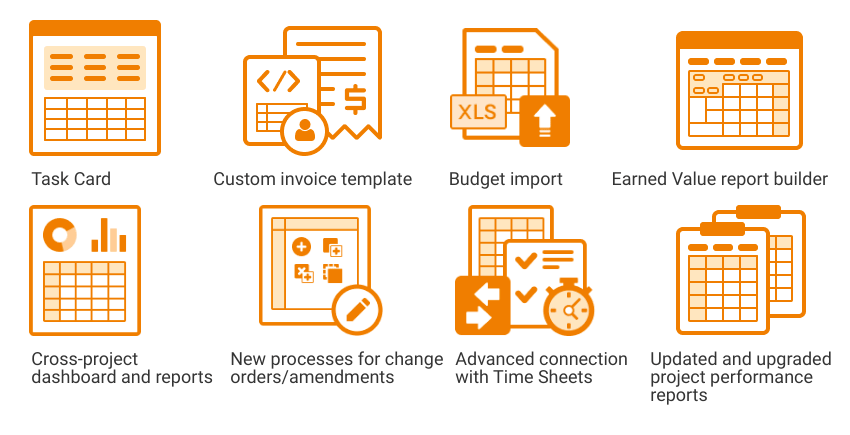
We constantly update and develop our system to meet clients’ expectations and provide the most advanced and user-friendly system for their needs. Here’s what’s new in Flex Databases Application Release 12. Common functionality Project Catalogue Larning Management System Pharmacovigilance eTMF – My Favorites – TMF Activities – TMF Master Copies – My Views Project Management […]
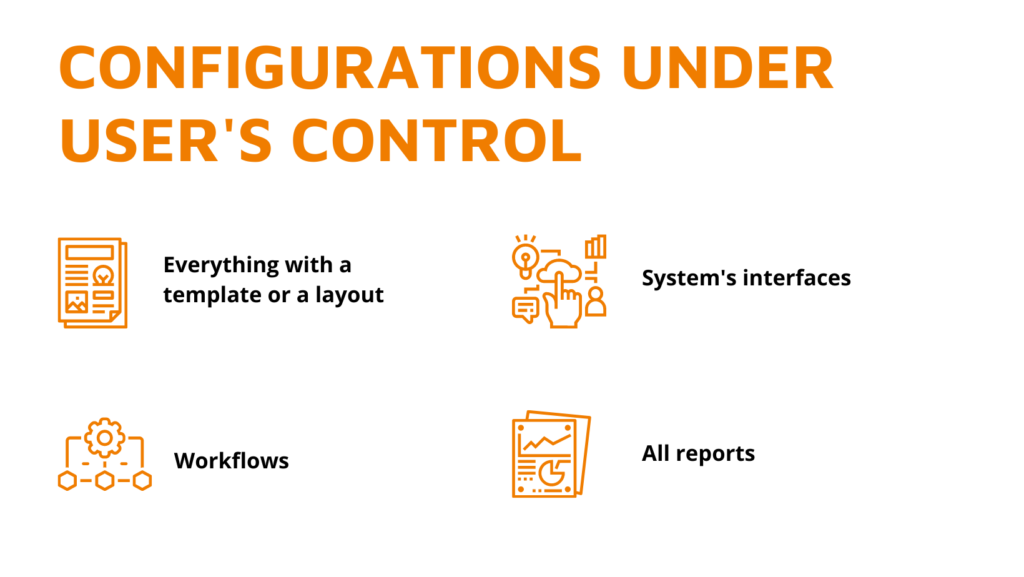
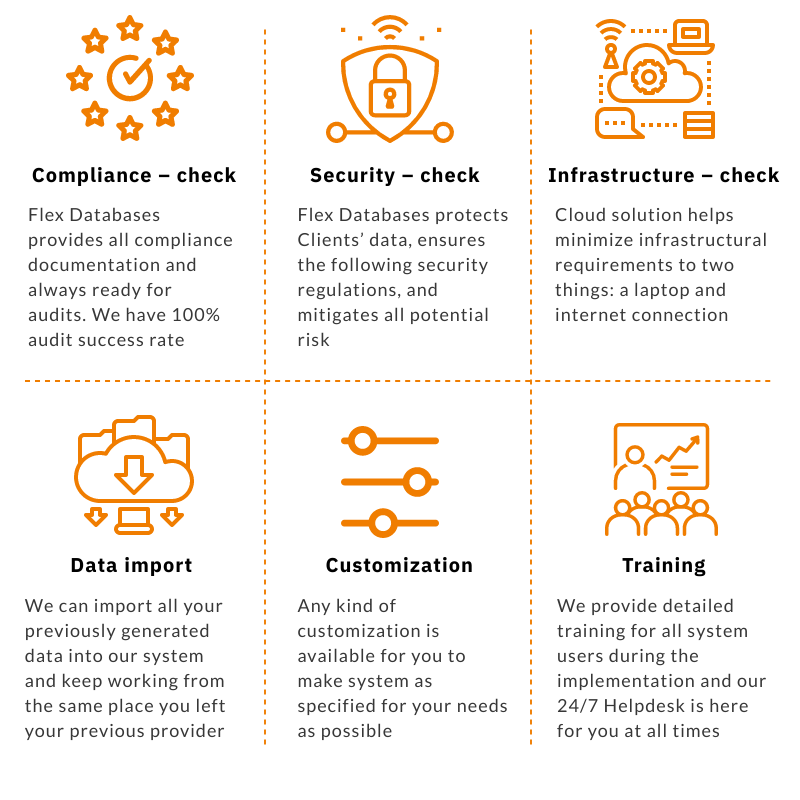
Changing your software provider is a massive burden since there were already many resources invested into picking your current one, implementation, etc. We talk to potential clients nearly 24/7 and 365, and we’ve heard all the concerns and troubles that exist. So, here is a six-step checklist of the most prevalent concerns before changing a software provider […]
At the very end of winter, Flex Databases signed a contract for CTMS and Pharmacovigilance module implementation with Italian Human & Digital CRO Exom Group. We’ve asked Luigi Visani, MD, President & CEO at Exom Group a couple of questions about the upcoming partnership: Why did Exom Group decide to pick Flex Databases as a provider? “Since its inception, […]
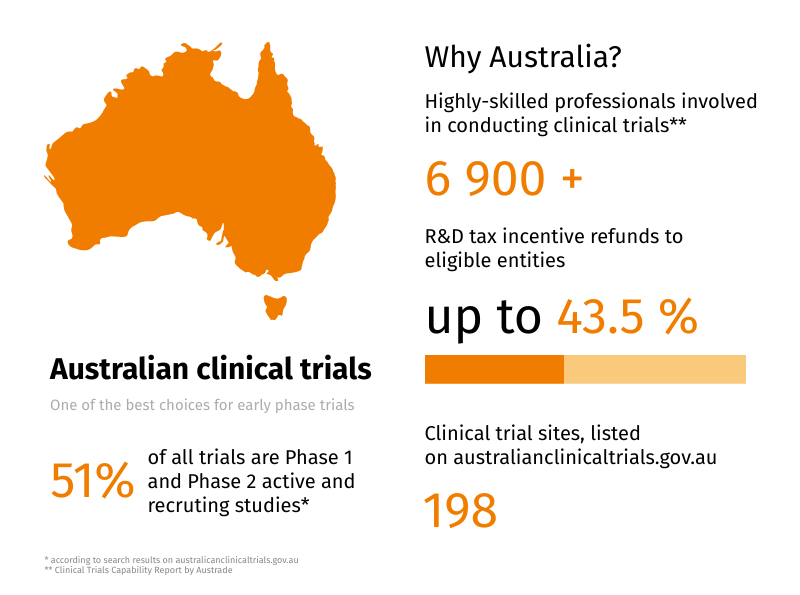
When industry experts talk about the role of Australia in worldwide clinical trials, many mention its perfect fit for early phase trials conduction. Despite the noticeable remoteness, the country has many advantages to offer for clinical trial sponsors all over the world. Let’s outline the biggest of them and see, why Flex Databases is a […]
At the very beginning of March 2021, Flex Databases signed a contract for eTMF implementation with Austrian clinical industry experts’ company Gouya Insights. We’ve asked Gouya Insights CEO, Ghazaleh Gouya a couple of questions about the new partnership: Why Gouya Insights decided to pick Flex Databases as a provider for TMF module? What do you expect to achieve […]