Blog Flex Databases
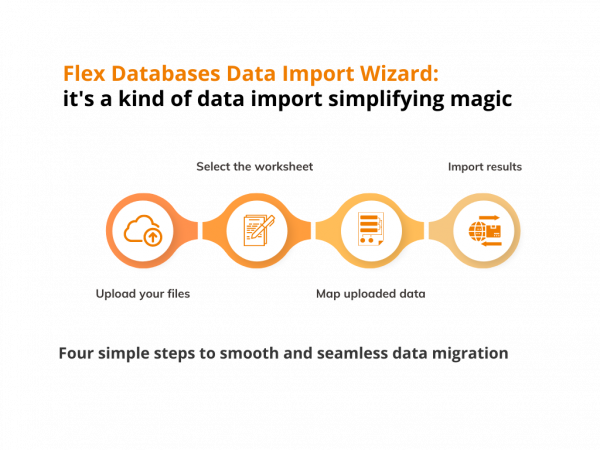
We have constantly been developing our eClinical solutions keeping simplicity as one of our products’ core values. At the same time, we strive to implement new tools that make our clients’ lives easier and the processes faster. It is a significant issue when migrating to a new system, and this fact pushed our developers to […]
At the very beginning of 2022, Canadian CRO Nutrasource selected Flex Databases as an eClinical provider to cover the entire clinical trial lifecycle with a single solution. “‘We are looking forward to the efficiencies that will be gained across our team with the implementation of Flex Databases allowing us to move to an single solution where we […]
We are extremely excited to announce that we start off 2022 with an offline event – SCOPE 2022! Evgenia Mikhalchuk, Director, Business Development & Marketing, and Vladimir Kolbatov, Product Director, will be there for you at booth #215. To schedule a demo or any other meeting prior to the event, please, message us at bd@flexdatabases.com After all […]
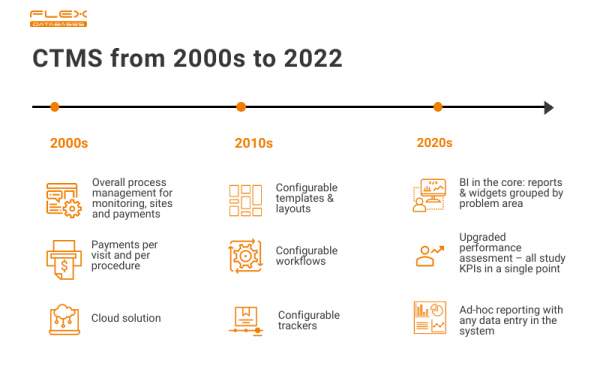
Insights on what makes the most impact on the project. Instant full picture in one interface. Sense of full control. Clinical Trial Management System (CTMS) is one of the most popular and widely used eClinical software applications. It’s been on the market for decades, and from simple process management, the concept grew in many different directions. […]
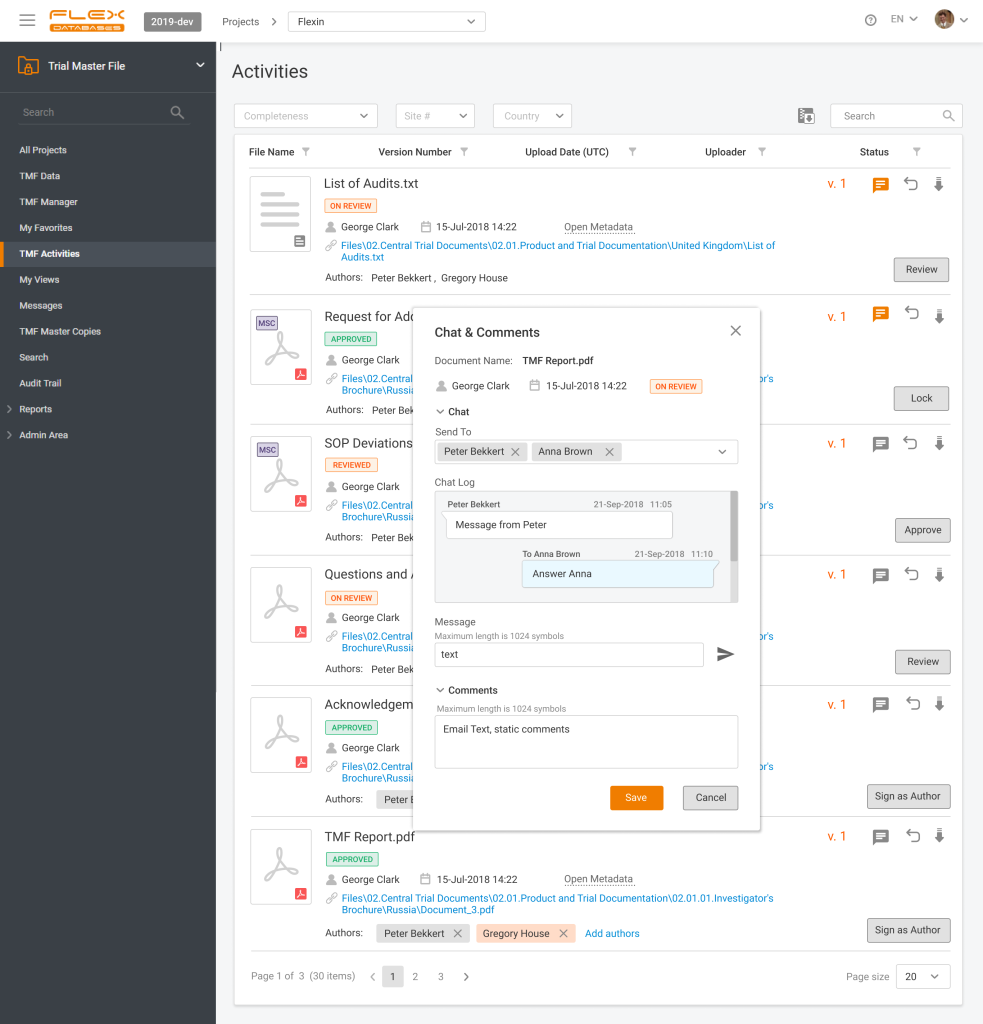
Have you ever missed a last-minute change in the file you’re working with? Do you lose track of thousands of messaging apps and emails? Do document review bugs you by being distributed into many channels, that you must align together? It’s not a problem anymore! With eTMF chat in Flex Databases eTMF everything, that you […]
Digitalization, or moving to a new software provider for whatever reasons usually comes together with resistance. You don’t want to change your processes, since there are a lot of employees to get on board with the change. However, you still want transparency, performance analysis, and all those benefits of having an electronic system in place […]
This summer Flex Databases and Faron, a Finnish biopharmaceutical company, signed a contract for eTMF implementation. We’ve asked Maria Lahtinen, Director, Supplier Management at Faron, a couple of questions about the new partnership: Why Faron decided to implement eTMF? What was the starting point for digitalization? Faron had two aims for the project – to have […]
Here’s the plan for the video: • What’s validation and why it is needed • Validation flow • What documents should a regulated company have after validation? • What Flex Databases provides and covers • What is left for a client to cover and how we can help • Clients’ feedback about Flex Databases validation […]
The system owner is the client’s employee responsible for the overall implementation and operation of a clinical trial management system on the client’s side. It is a primary contact for us as a vendor and a driver of change in the company. In this video, we discuss the role of the system owner, necessary skills and background, […]
This fall BioClever, Spanish full-service CRO, and Flex Databases signed a contract for eTMF, Project Management & Budgeting and Time Sheets & Utilization implementation. We’ve asked Jordi Cantoni, Director at BioClever a couple of questions about the new partnership: Why BioClever decided to implement eTMF, Project Management & Budgeting, Time Sheets & Utilization? The main reason to decide […]
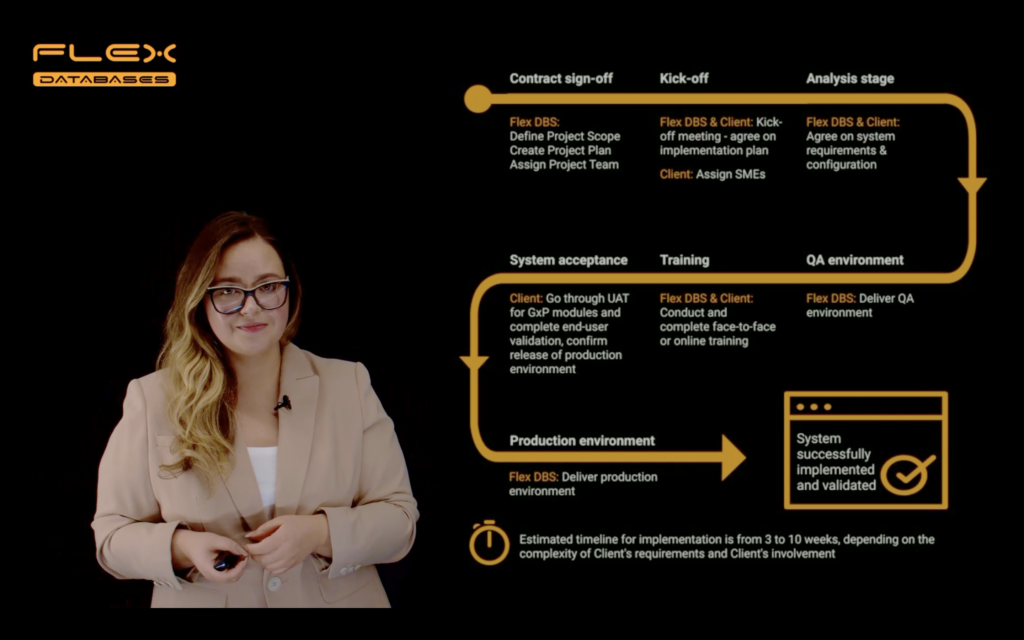
We can deliver a fully functional system in the timeframe from 3 to 10 (typically around 5) weeks, depending on the Client’s involvement and the complexity of the Client’s requirements. This video is an overview of how the implementation process goes. Timecodes: 00:09 About Flex Databases 00:20 Plan for this video 00:26 Typical implementation scope 00:41 Flex Databases implementation […]
What are the components of a typical eClinical software pricing and how do we at Flex Databases approach it? Here’s an answer. Timecodes: 00:14 About Flex Databases 00:26 Plan for this video 00:36 Typical budget structure for SaaS eClinical system 01:37 Flex Databases cost structure 02:39 What if I need a new function or customization? 03:01 Ideas Lab 03:38 How we grow […]