Blog Flex Databases
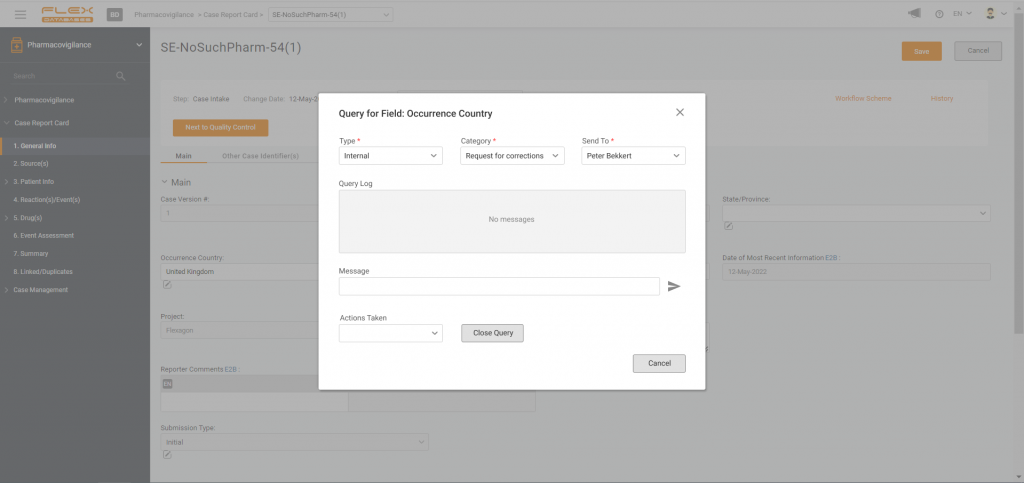
In this video we talk about the way Flex Databases Pharmacovigilance module helps to save time on case intake. Video Duration: 03:28
The travel season for 2022 is on – and this time we meet in Chicago, our kind of town. Our best of the best will be attending DIA Annual Meeting 2022 – Evgenia Mikhalchuk, Director, Business Development & Marketing, Vlad Kolbatov, Product Director & Natalia Blagodarova, Director, Operations. To meet those fine people you could […]
Earlier this year Sun Pharma Advanced Research Company (SPARC) selected Flex Databases to be a full-scale eClinical vendor, purchasing eTMF, CTMS and Project Management & Budgeting solutions. We’ve asked Maya Bhattacharya, Manager-I Business Continuity (Implementation Lead) a couple of questions about the upcoming partnership: Why SPARC decided to implement eTMF, CTMS and PM&B? What was […]
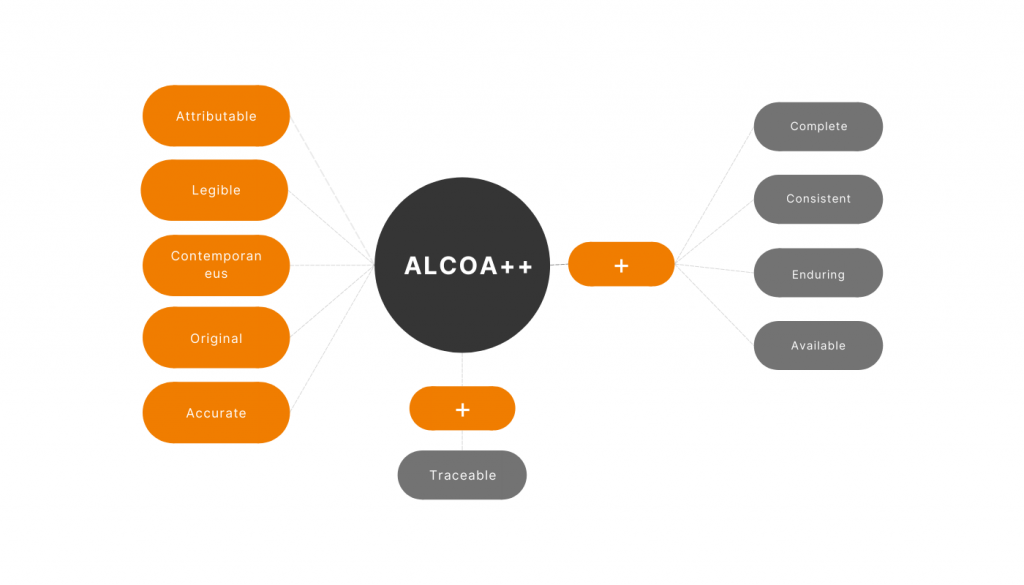
Data integrity has always been a critical point during the development of eTMF systems. One of the industry’s latest standards is ALCOA++, and our eTMF module is 100% compliant with ALCOA++. Let’s see what advantages it brings to our clients. A – Attributable In our eTMF module, all actions conducted with each item are caught, […]
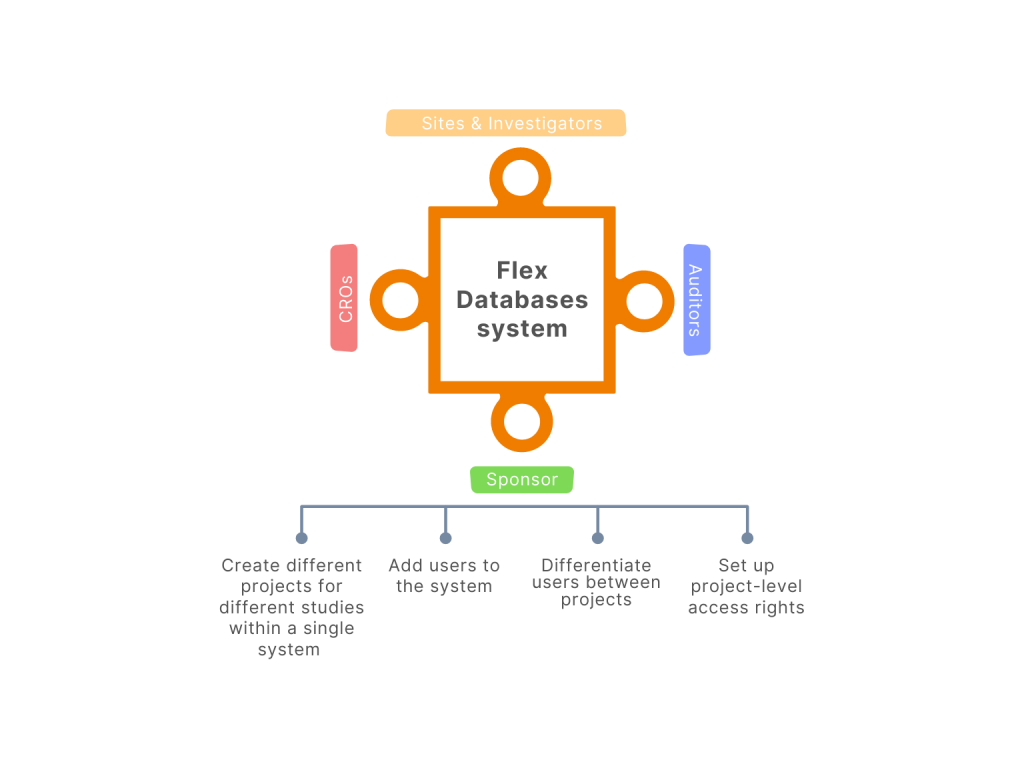
Are you the only one who needs access to the system? In clinical trials, we often need to collaborate with a lot of partners for each trial or sometimes even to execute different tasks. And the more partners we have – the more difficult it appears to manage all the processes. Moreover, many people think […]
What was always a problem? Every single person connected to clinical trials has always been dreaming to have one button to click Every person connected to clinical trials has always dreamed of having one button to click that could allow see all the information about the trial at once. Everybody dreamed about the place where […]
Our QA Director Anna Petrovskaya will attend Virtual European QA Congress 2022, organised by the RQA, as a speaker. Date: 11.05.2015 Topic: What is the Audit Trail for the Auditor? Time: 14:00 You can find more detail and book your ticket here.
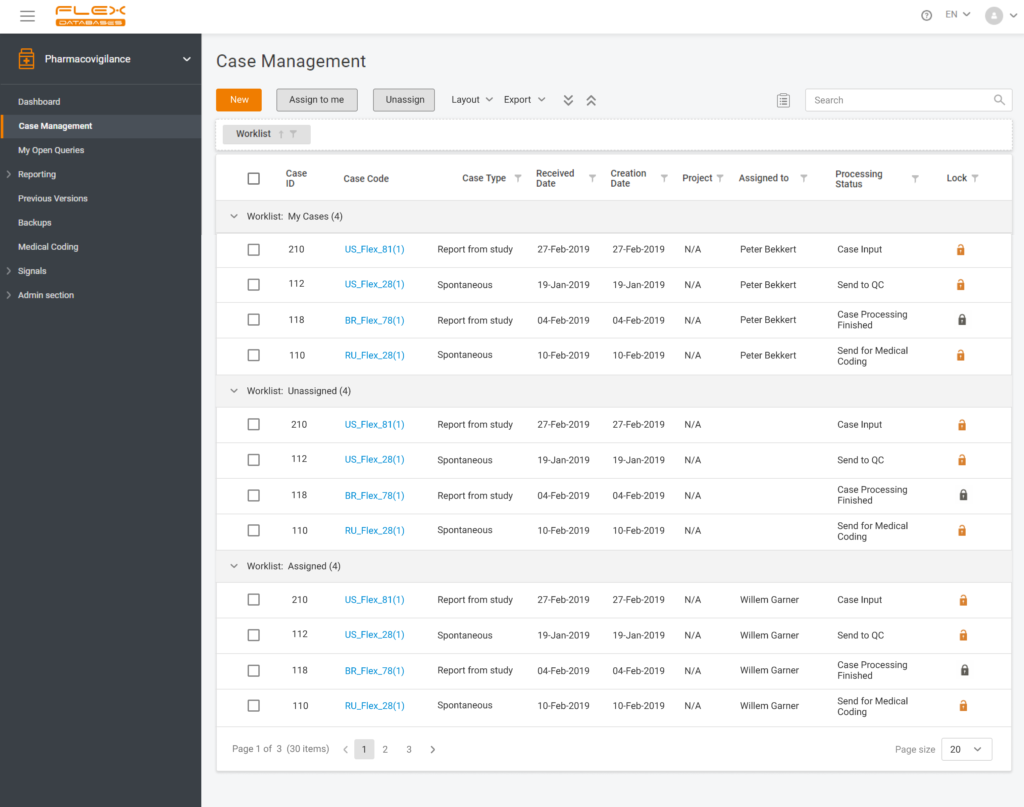
Each step of Flex Databases is always done with one thought in mind – to save time and money for our clients. That’s why to save your time and give you a deeper understanding of how our Pharmacovigilance module work, we are happy to answer the six most frequently asked questions about the system. How […]
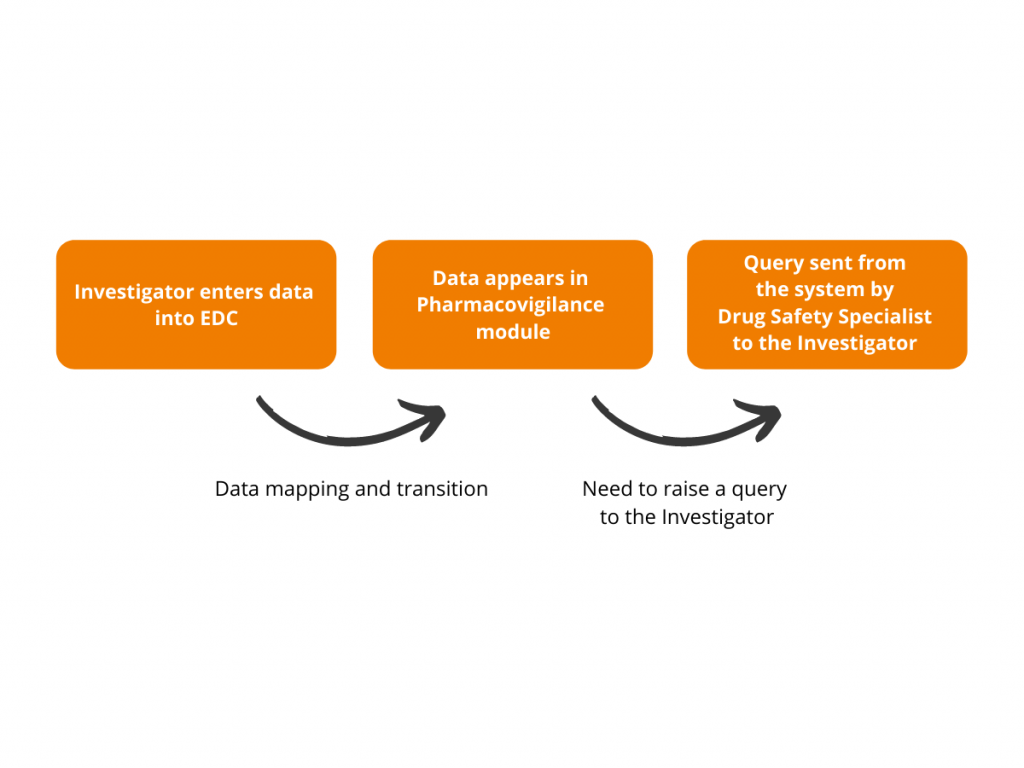
Many years since the appearance of different platforms and systems for clinical trial operations, there has been a question about making all the systems work synchronized. For most parts of the platforms and systems, it occasionally became possible, though Drug Safety Specialists still have to lead their pharmacovigilance processes independently. Having the objective to streamline […]
Clinical Trial Management System (CTMS) is an essential tool for any modern company, engaged in clinical trial process somehow. We’ve decided to compile an Ultimate Guide about Flex Databases CTMS and answer the biggest questions a person could have on the subject: from what is CTMS to what’s coming next. Table of contents: Chapter 1: […]
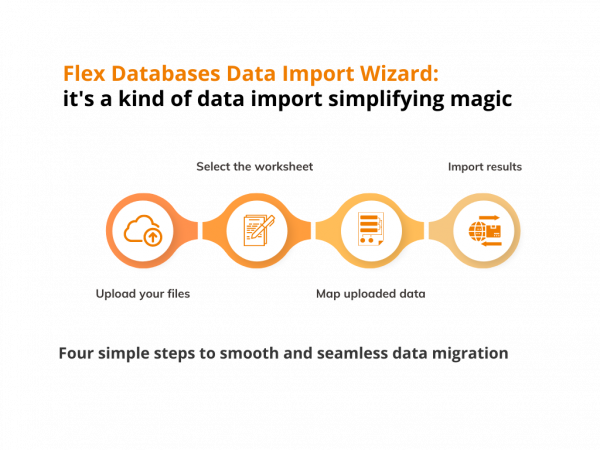
We have constantly been developing our eClinical solutions keeping simplicity as one of our products’ core values. At the same time, we strive to implement new tools that make our clients’ lives easier and the processes faster. It is a significant issue when migrating to a new system, and this fact pushed our developers to […]
At the very beginning of 2022, Canadian CRO Nutrasource selected Flex Databases as an eClinical provider to cover the entire clinical trial lifecycle with a single solution. “‘We are looking forward to the efficiencies that will be gained across our team with the implementation of Flex Databases allowing us to move to an single solution where we […]