Blog Flex Databases
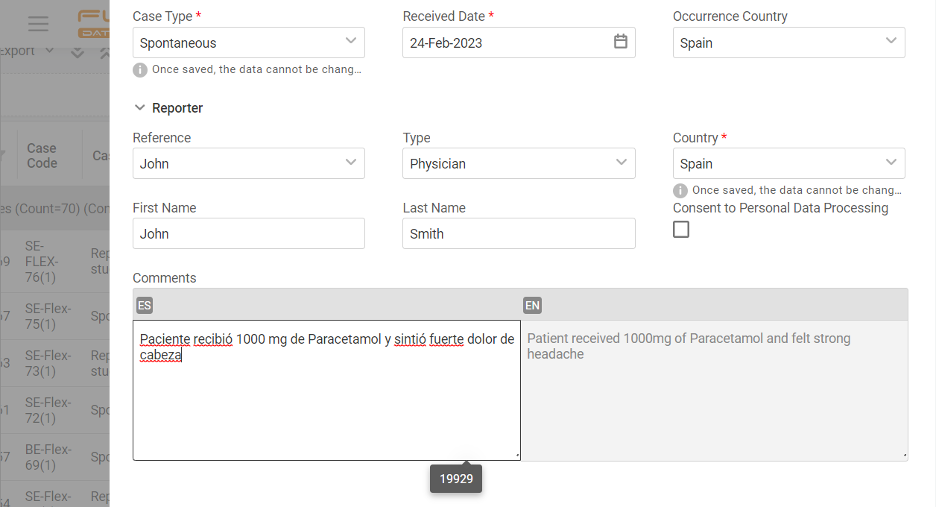
If you operate globally, you must comply with local language requirements, which also apply to pharmacovigilance reporting. Imagine that you must manage cases & report in English, Spanish, and French. How would you do that? The apparent answer many providers usually offer is multiple inputs of the same cases. Drop that! With Flex Databases Pharmacovigilance, […]
As we grow not only by numbers but also by the number of different nationalities in our company, we focus more on our internal cross-cultural communication processes. Sometimes we are all very different, and it is true, but we are all the same when it comes to delivering the best result and fostering eco-communication at […]
There are three big questions in any industry, including pharmaceuticals & clinical trials: Artificial intelligence is one of the new answers to all of them. Here are a few examples: When it comes to saving time, AI is here to take over data processing – fishing for answers to most minor questions in huge data […]
Implementing Quality Management System might be a struggle. You have it perfect on paper, but you must comply with the harsh reality of your software vendor’s system logic. Well, it’s not a problem anymore with Flex Databases QMS. In our most recent update, we’ve made every process within QMS fully configurable. You get the LEGO blocks […]
We’re excited to announce a new client – a US-based biotech iRenix Medical. Earlier this year, iRenix team selected Flex Databases as an eTMF provider. We’ve asked Stephen J. Smith, MD, Founder and CEO at iRenix Medical a couple of questions about the upcoming partnership: Why iRenix Medical decided to implement eTMF module? What was the […]
We’re excited to kick-off events of 2023 by visiting SCOPE 2023 in Orlando, the US. To meet with our one and only Business Development Director Evgenia Michalčuk-Vráblík and Product Director Vlad Kolbatov, simply step by our booth #728. Can’t wait to meet you all!
We’re starting our 2023 with some great news about a new client – Belgian Anima Research Center selected Flex Databases as a LMS & QMS provider. We have asked Linde Buntinx, Chief Development Officer at Anima, a couple of questions about the upcoming partnership: Anima Research Center is continuously innovating and optimizing their clinical study […]
Flex Databases launches Innovation Unit within Teknokent Antalya to further develop advanced solution for data-informed decisions in drug development. We’ve ended 2022 on a high note – by being accepted into Teknokent Antalya as residents with our Innovation Unit. Flex Databases Innovation Unit aims to further develop our Powerful Datahouse to address the needs of […]
Software selection and assessment is a complicated and sometimes confusing process, especially if you have to pick a provider in a hurry or if it’s your first time doing so. We’ve gathered a short list of mandatory criteria for you to check out – and have an excellent start to your EDC selection journey. Investigators […]
Clinergy Health Research recently selected Flex Databases as Pharmacovigilance software provider. We’ve asked Suyh Lin, Clinical Project Manager at Clinergy Health Research a couple of questions about the upcoming partnership: PV system and digitalization was implemented due to one of our client’s needs, and considering the expectation of an important number of safety cases to […]
Clinical trials, as everything else in the world, is undergoing a heavy shift to process digitalization and automation. It has its ups and downs, but the future looks bright – we can minimize human error, optimize, and automate our processes and save money. Today we’ll talk about how exactly you can move from spending money […]
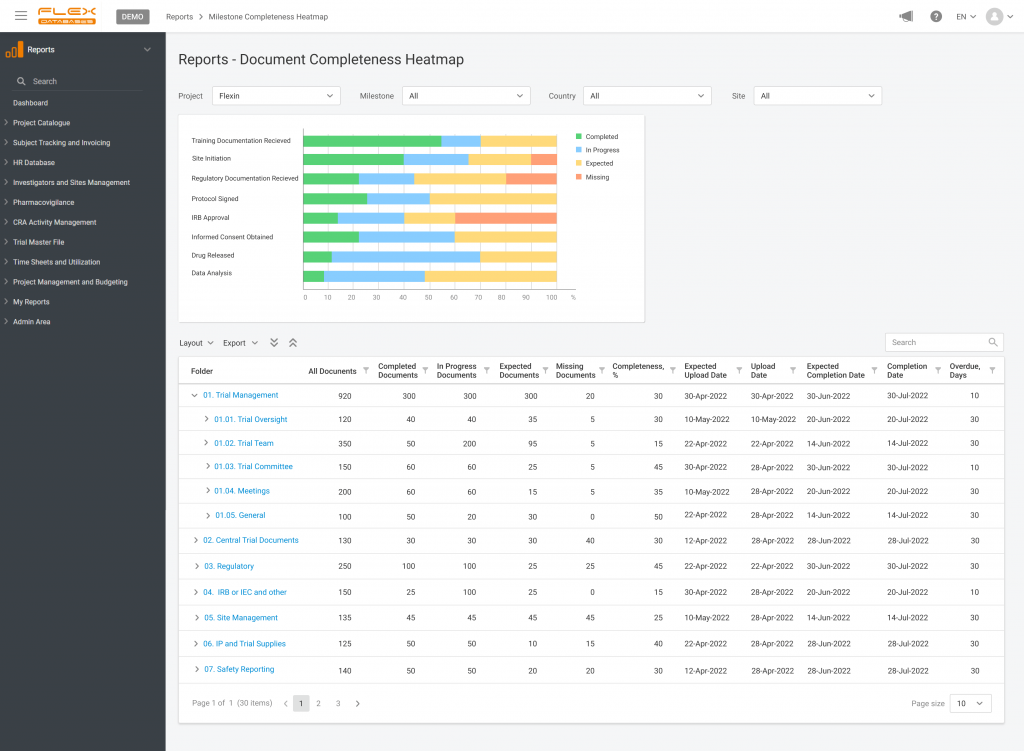
We kept six main questions in mind during the development of milestones. Do you want to know the answers? Milestones help us to: Plus, milestones demonstrate the trial’s success and completion in achieving financial targets. When you create a project in the system and enter its name, you can add the milestones. Doing that allows […]