Blog Flex Databases
Deloitte, a renowned leader in professional consulting, and Flex Databases, a trailblazer in flexible electronic systems, have officially announced their strategic alliance, signed at the end of year 2023. This collaboration is set to transform the Life Sciences sector by leveraging Deloitte’s expertise in process management, validation, onboarding, and consulting, alongside Flex Databases’ state-of-the-art electronic […]
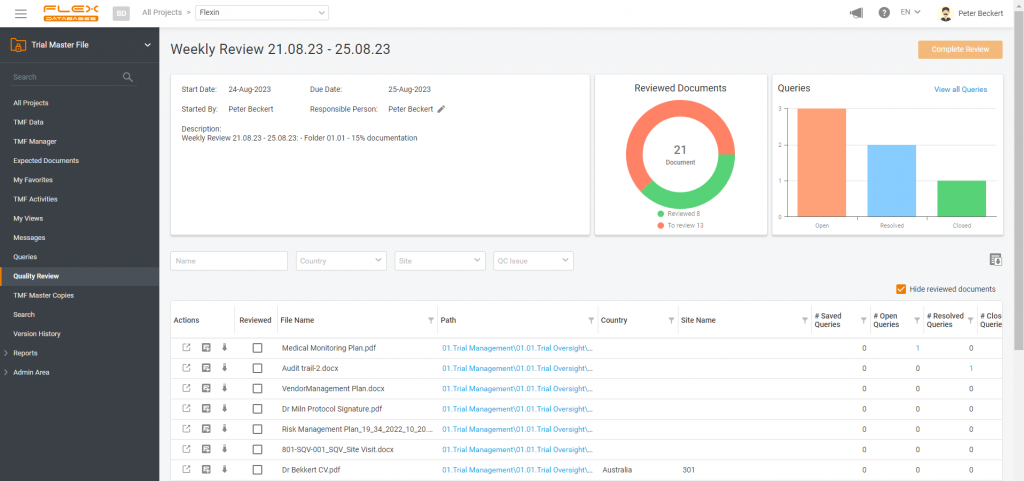
Periodic TMF reviews automatically documented. Quality and completeness under control! Let’s address an intriguing issue in the clinical trial landscape. It appears that while one third of sponsors are less than satisfied with the quality of clinical trials, an overwhelming 90% of CROs are confident in their high-quality delivery. The solution might lie in implementing […]
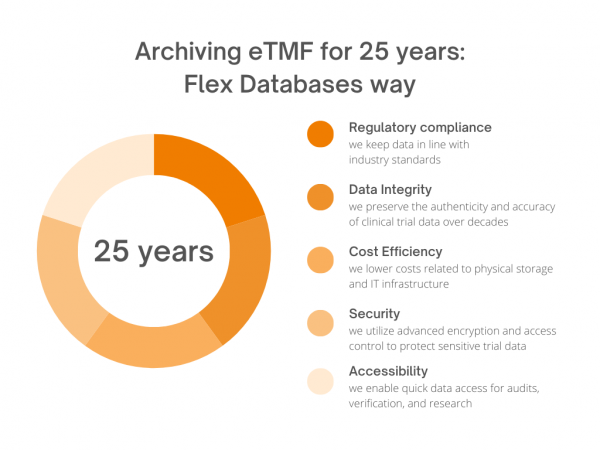
Good Clinical Practice guidelines require clinical trial documents to be kept for at least two years after the last marketing application approval in an ICH region. These documents may need to be stored longer if regulations or the sponsor require it. The EU Clinical Trial Regulation (CTR) 536/2014 demands that the content of the clinical […]
Late November we’ve hosted a joint webinar with Deloitte on how you can and why you should consider digitalizing Quality Management System. A joint webinar on quality digitalization with Vera Valesova, PhD, Senior Manager at Deloitte Risk Advisory/Life Science&Healthcare unit. Vera joins the stage together with our QA Director, Anna Petrovskaya, a well-recognized quality professional […]
November 21st, 17.00 CET we’ll have a joint webinar on quality digitalization with Vera Valesova, PhD, Senior Manager at Deloitte Risk Advisory/Life Science&Healthcare unit. Vera will join the stage with our QA Director, Anna Petrovskaya, a well-recognized quality professional and RQA speaker. Here are the main questions that Anna and Vera are going to cover […]
We’re excited to announce our participation in 11th Annual Outsourcing In Clinical Trials Nordics 2023, October 24 – 25, Copenhagen, Denmark. Not only can you meet our brilliant team members during the event, but also encounter a chance to hear Evgenia Michalčuk-Vráblík, Global Director of Growth, give a talk titled “Seamless Digital Collaboration: Uniting Vendors […]
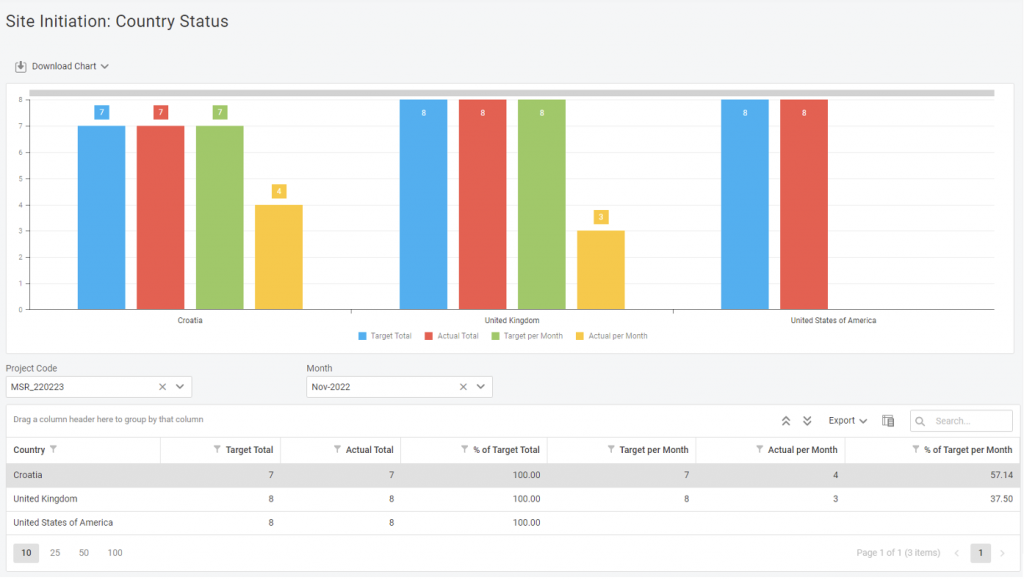
Nowadays, there’s no such thing as too much optimization in team or process management. That’s why we decided to get you another step ahead with our KPI reports – multi-component reports that’ll help you focus everything essential on one screen. We’ll look at Site Initiation and Enrollment reports for today’s exercise. In one report, you […]
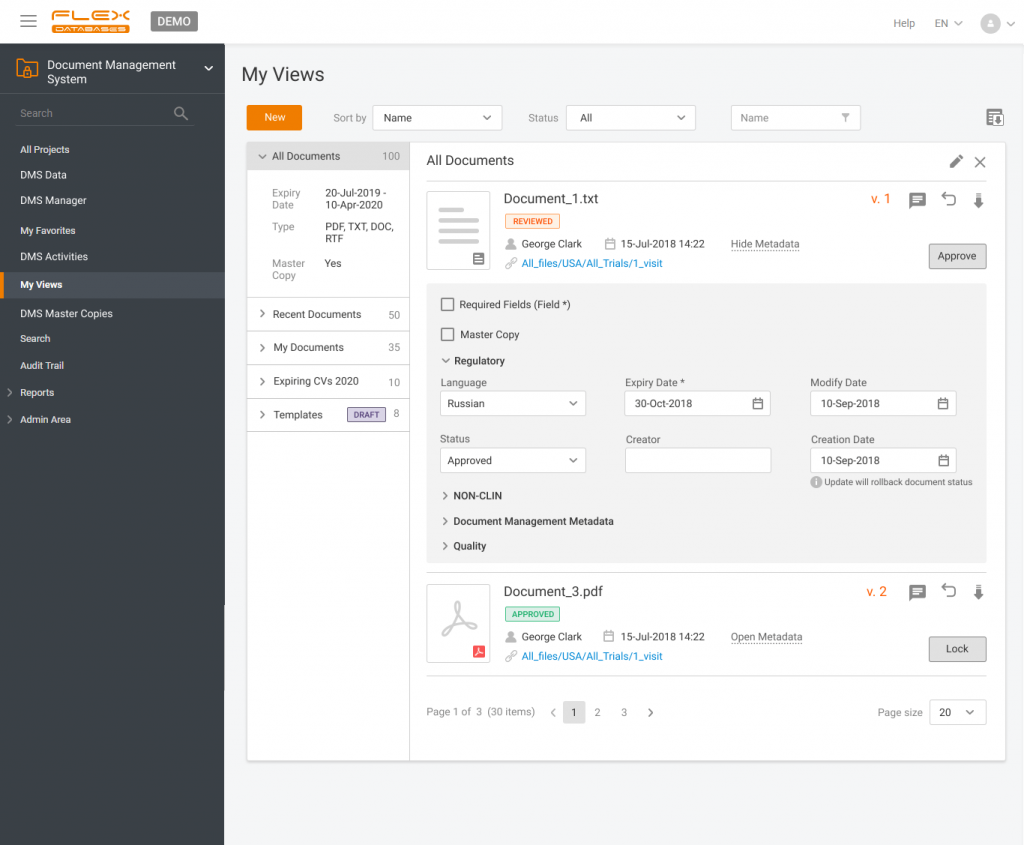
Document management & storage is rarely considered as something complicated, however, it’s a backbone for everything. When it’s done wrong, you’ll experience process delays, incomplete or missing documents, and other data integrity & completeness issues. Flex Databases DMS is done exactly right – and here’s a quick overview of manager & user processes, that get […]
Earlier this summer, WSG – Westdeutsche Studiengruppe GmbH selected Flex Databases as a CTMS and eTMF provider. We’ve asked Michael Städele, Director, Business Development & Operations a couple of questions about the new partnership: Over the last years, we are facing an increasing number of trials, employees, and study sites. In addition, more of the […]
As always, DIA 2023 was mostly about the people we meet & talk to, but there is a big second value to it – the talks, the ideas and insights that we get during the conference. Let’s take a look at what’s hot in Boston! We can’t wait to be back in 2024!
We always say that integration of the systems is one of the keys to success. That’s why we pay a lot of attention to API at Flex Databases. Flex Databases has become a member of CRISI – The Clinical Research Interoperability Standards Initiative, developing a comprehensive library of standard APIs. We are proud to be […]
Our BD&Marketing Director Evgenia Michalčuk-Vráblík has recently set down with LifeScience Review reporter to talk about the No-Code approach that Flex Databases is leading in CTMS world and how it benefits our client’s business operations. The interview followed the reward that Flex Databases received as one of Top-10 CTMS providers in 2023. While maintaining compliance, […]