Blog Flex Databases
Metronomia, a leading Data Management and Biostatistics CRO in EU, has selected Flex Databases’ eTMF module to support one of its biotech clients. Simon Rückinger, Managing Director at Metronomia Clinical Research GmbH, explained their decision and what they expect from the partnership. “We needed a flexible and simple eTMF solution that could meet the specific […]
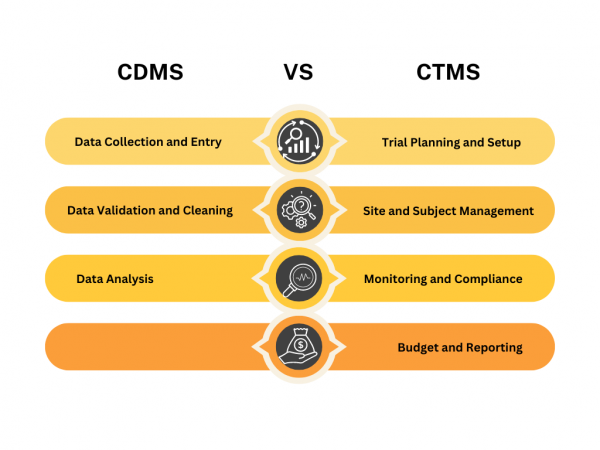
Clinical Data Management Systems (CDMS) and Clinical Trial Management Systems (CTMS) are two key tools that play crucial roles in this process. While they might sound similar, they serve distinct purposes and offer different functionalities. What is a Clinical Data Management System (CDMS)? A Clinical Data Management System (CDMS) is a specialised tool designed to […]
A regulatory binder is crucial for managing clinical trial documents, it ensures compliance with regulations, and makes audits easier. It helps keep important documents organized and readily accessible for trial monitors, auditors, and regulatory authorities, and serves as a useful reference for the research team. While it’s not legally required, maintaining a regulatory binder is […]
Trial master files (TMFs) are essential for completing clinical studies, but managing TMF content can be very complex. Electronic trial master file (eTMF) systems represent the next step in clinical trial document management and are becoming an integral part of future clinical research. What is an eTMF? An electronic trial master file (eTMF) is a […]
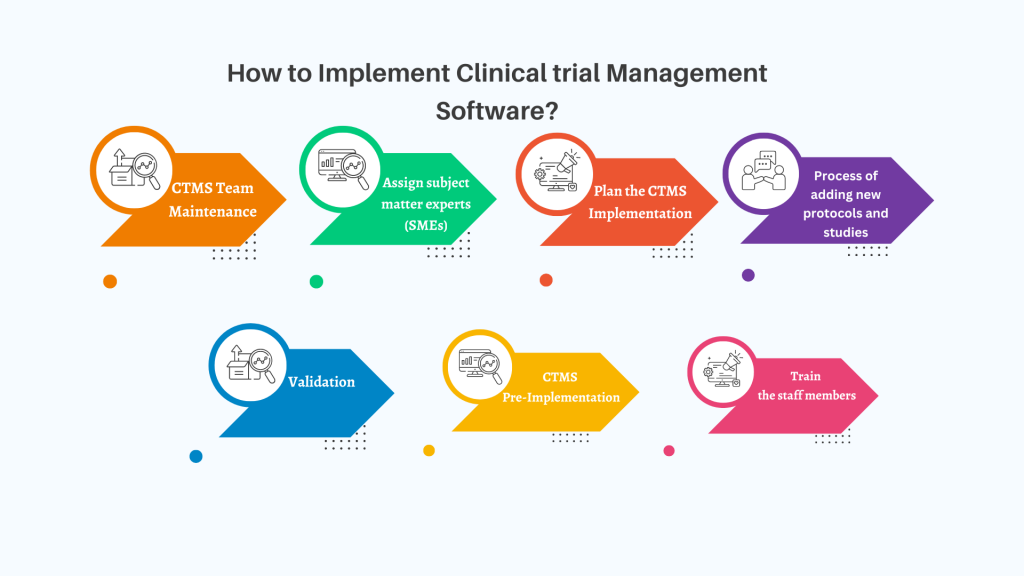
Clinical trials play a crucial role in medical progress, yet effective management is essential to maximize their impact. Administrators must craft a thorough strategy to enhance how trials are managed. Here are five strategies to transform clinical trial management, providing administrators with the tools and insights needed to navigate healthcare research challenges effectively. Simplify planning […]
Effective site selection and management are critical components of successful clinical trials. Choosing the right locations and overseeing their operations can significantly impact the trial’s timeline, budget, and ultimately, its success in delivering reliable data. This article explores essential strategies for optimizing site selection and management, highlighting key considerations, practical tips, and the role of […]
Handling a Trial Master File (TMF) can be tough for clinical teams, but it doesn’t have to be. Old systems with outdated file sharing methods or homemade systems with spreadsheets or paper can make managing sensitive trial data risky. Over the past decade, electronic Trial Master File (eTMF) systems have helped many biopharma companies and […]
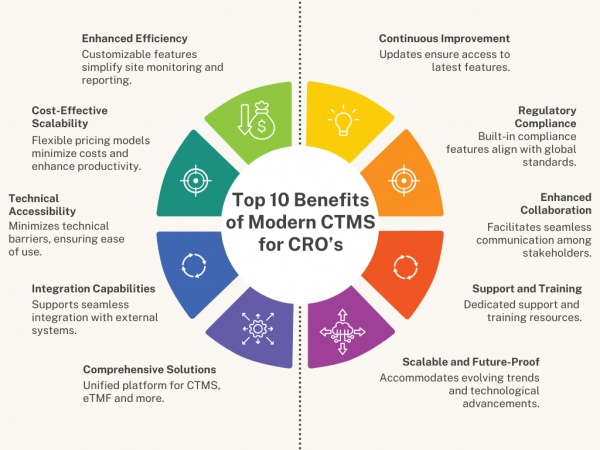
Clinical Research Organizations (CROs) operate in a dynamic industry where efficiency and adaptability are critical. As the global clinical trials market expands significantly, with forecasts predicting substantial growth, CROs face increasing demands to manage multiple trials efficiently across diverse projects and stakeholders. Legacy systems, still prevalent among CROs according to recent surveys, often fall short […]
A Clinical Trial Management System (CTMS) is software designed for the Life Science industry. It helps manage processes and handle substantial amounts of data related to clinical trial management and clinical study management. CTMS typically handles the planning, preparation, execution, and reporting of clinical trials. It focuses on maintaining updated contact information for participants and […]
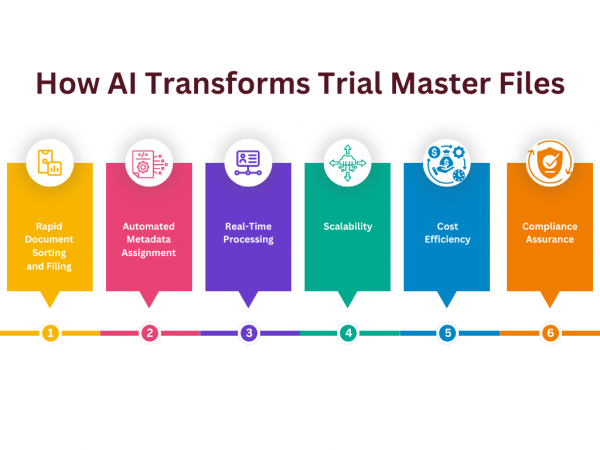
In today’s busy world of clinical research, managing documents efficiently is crucial. Imagine being able to organize hundreds of documents in just minutes. Here’s how Flex Databases’ AI-powered Trial Master File (TMF) system makes this possible. From Chaos to Order in a Few Clicks The process is remarkably simple. Users can drag and drop a […]
We’ve always been proud of our Quality Management System, which has been recognized by our clients during numerous audits and has helped us become a trusted vendor to regulated companies all over the world. Now, we have documented evidence – the ISO 9001:2015 certificate. Flex Databases’ ISO 9001:2015 certification demonstrates its dedication to providing a […]
Quality management in clinical research is crucial to ensure the integrity, reliability, and validity of the data collected. This process encompasses all activities that ensure the study is conducted in compliance with regulatory requirements, guidelines, and best practices. Adding QMS strategy databases in your organization can help your processes by miles. For one, implementing a […]