Blog Flex Databases
Clinical Trial Management Systems (CTMS) and Electronic Data Capture systems are critical to the effective management of clinical trials. By integrating CTMS and EDC, organizations can streamline processes, enhance data quality, and improve overall efficiency. Here are five key tips to ensure a successful CTMS and EDC integration. Effortless Integration of Flex Databases CTMS with […]
A new round of our traditional meetings took place in October. This time, we gathered our beloved users to discuss the use of eTMF – from everyday activities to audit preparation. We reviewed how newly released features, such as duplicate search, quality review, and metadata notifications, influence TMF workflows. It appeared not everyone was using […]
We’re excited to announce that Flex Databases will be participating in this prestigious event from November 4–6, 2024. Join us to explore our innovative solutions! Connect with Kristina Kojoushko at the event to learn more about our services, including eTMF, CTMS, Pharmacovigilance Software and more. 📅 Schedule a meeting with Kristina at kristina.kojoushko@flexdatabases.com or reach […]
Did you miss our recent webinar on Quality Digitalization? The recording is now available! In this insightful session, Anna Petrovskaya, QA Director at Flex Databases, and Vera Valesova, PhD, Senior Manager at Deloitte, explore the critical aspects of transforming your QMS into a digital solution. Key topics covered: Watch the full webinar and gain valuable […]
October, 2024 – Agenus Inc. (NASDAQ: AGEN), an immuno-oncology company with a strong pipeline of immune modulating antibodies, together with Flex Databases announce the successful implementation of Flex Databases’ Clinical Trial Management System (CTMS) and electronic Trial Master File (eTMF) solutions, including a new AI-driven feature for automated document filing and metadata assignment. This partnership […]
In October 2024, the U.S. Food and Drug Administration issued a final guidance titled Electronic Systems, Electronic Records, and Electronic Signatures in Clinical Investigations: Questions and Answers. https://www.fda.gov/media/166215/download This guidance provides recommendations on the use of electronic systems, electronic records and electronic signatures in food, medical product, tobacco product, and new animal drug clinical investigations. […]
We had the pleasure of sitting down with Martin Krauss, Co-Founder and Managing Director of FGK CRO, to discuss how Flex Databases has supported their clinical research operations over the past 3.5 years.In this interview, Martin shares: • Why FGK CRO initially chose to partner with Flex Databases.• How our CTMS, TMF, and LMS solutions have transformed […]
We are thrilled to share several key enhancements that will streamline your workflows and improve data management. Enhanced Duplicates Search Our Duplicates Search feature has received a significant upgrade! The system now identifies 100% duplicate files from your Inbox by checksum, ensuring cleaner data management. When files are moved from the Inbox to any folder […]
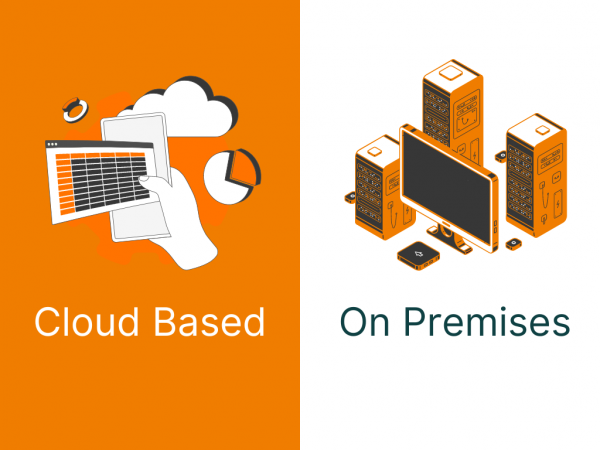
Two popular models dominate the Clinical Trial management System landscape: Cloud-based and On-premises CTMS. Both have their merits, but choosing the right option depends on several factors, including cost, scalability, security, and operational requirements. This article will explore the differences between Cloud-based Clinical Trial Management System and on-premises CTMS, helping you determine which solution best […]
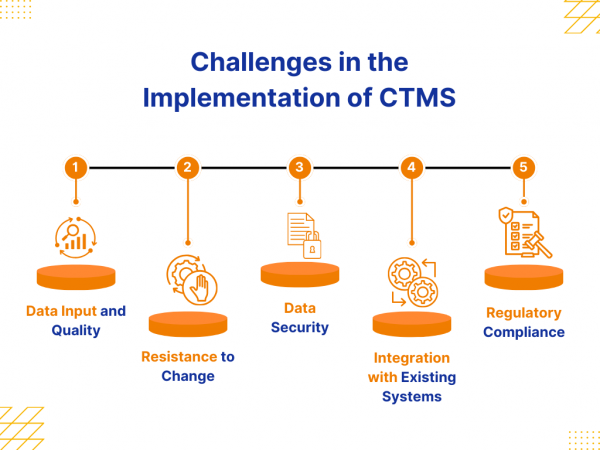
Implementation of a Clinical Trial Management System (CTMS) can streamline operations, improve data management, and enhance trial oversight. However, the process also brings a range of challenges that must be addressed to achieve a successful implementation. These challenges often stem from technical and human factors, including data quality, system integration, and regulatory demands. Identifying these […]
We are excited to share that Flex Databases has been named a Top Clinical Management Provider for 2024 by Life Science Review. This prestigious recognition highlights our strong reputation and the trust placed in us by both customers and industry peers, reflected in numerous nominations from Life Science Review subscribers. This award comes at a […]
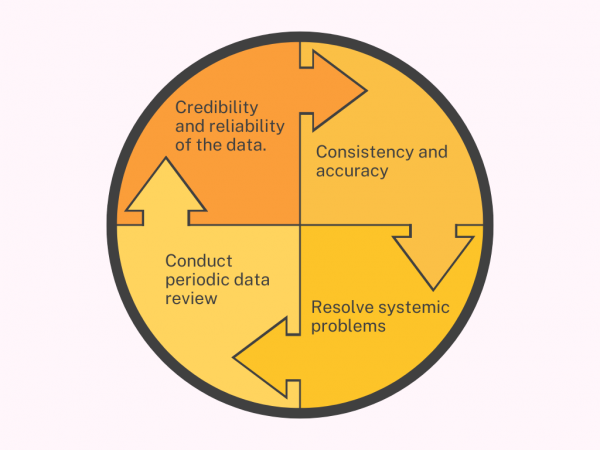
Clinical trials provide essential information on the safety and effectiveness of drugs. Sponsors ensure that investigators follow protocols and collect and report quality data. Quality in clinical trials means that the product or service meets applicable regulations and customer requirements. A Quality Management System (QMS) in clinical research ensures compliance with ethical, regulatory, and GxP […]