Blog Flex Databases
At Flex Databases, we believe the best solutions are built together. That’s why we created Flex Databases User Groups – a unique meetings where our clients and their end-users come together to: Our User Groups are more than just meetings – they are a space for open dialogue, collaboration, and innovation.By listening to your experiences, […]
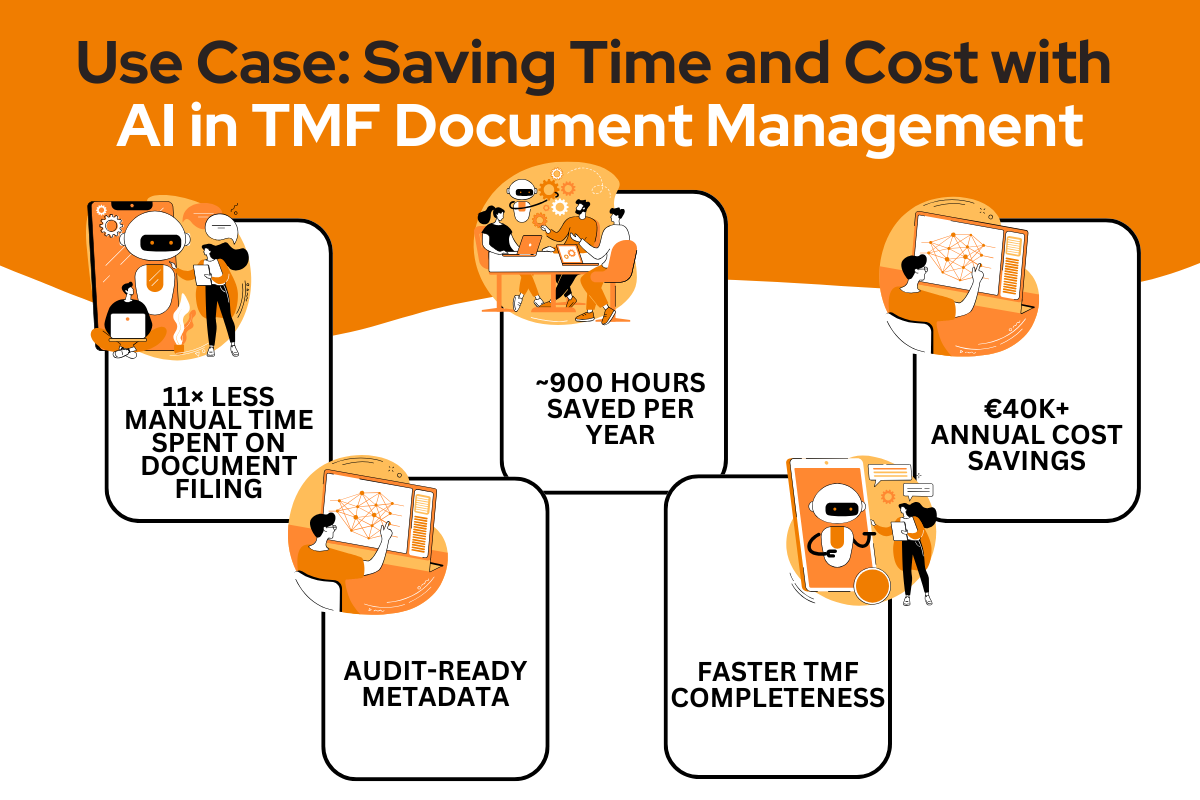
A mid-sized CRO managing 9,500 TMF documents annually faced increasing challenges with filing and metadata assignment. Each document required an average of 5–6 minutes to process manually. The Problem Manual document upload and metadata entry amounted to: This process also posed risks of human error and inconsistency. The Solution The CRO implemented an AI-powered eTMF […]
Nowadays the relationship between sponsors and contract research organizations (CROs) has evolved. What once was a simple outsourcing arrangement has become a strategic partnership, where both parties are expected to operate with full transparency, speed, and strict adherence to regulatory standards. Sponsors are no longer satisfied with periodic updates and disconnected spreadsheets. They want real-time […]
At Flex Databases, we’re on a mission to make a real impact in the Life Sciences industry with our CTMS, eTMF, and other systems for Life Sciences. Our platform supports Clinical Research Organizations and pharmaceutical companies across the globe in managing the complex world of clinical trials with greater clarity and efficiency. Now, we’re looking […]
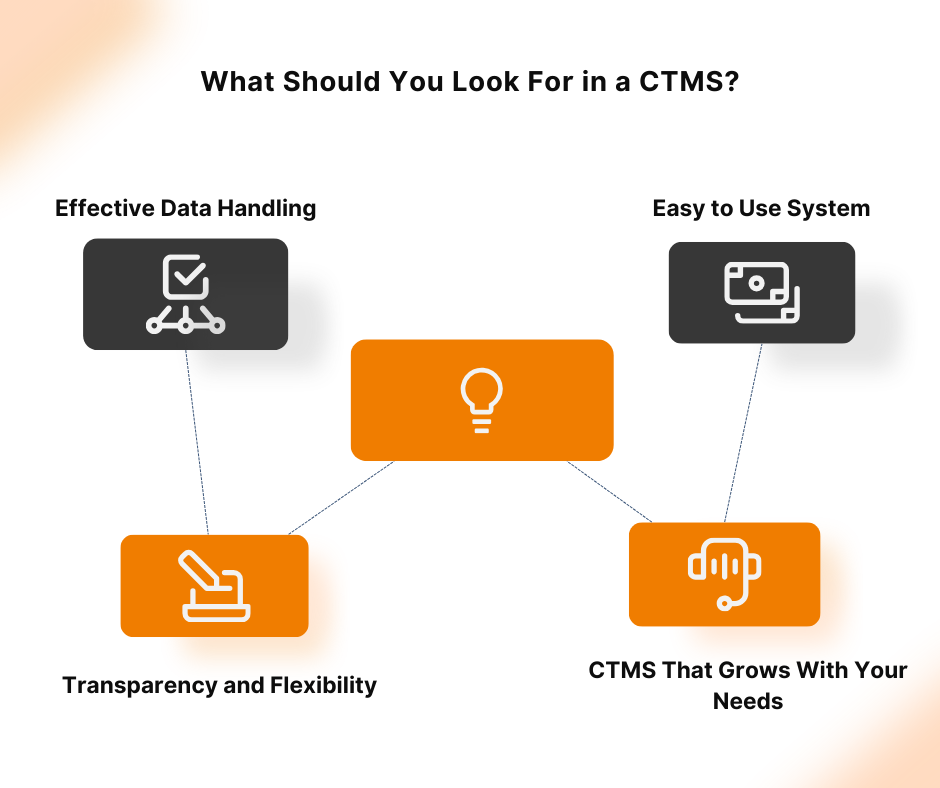
Clinical trials are essential for developing new medical treatments, yet they often face challenges like complex protocols, regulatory compliance, and data management issues. Implementation of a Clinical Trial Management System (CTMS) can address these challenges by centralizing data, automating workflows, and ensuring compliance, thereby enhancing efficiency and reducing errors. The adoption of CTMS solutions is […]
📢 Missed our webinar? 🎥 Watch the recording to see how SOLTI #cancerresearch streamlined trial management with Flex Databases! Our expert panel shares insights on CTMS selection, implementation, and daily use – plus a live mini-demo! In this session, we cover:🚀 Key triggers for adopting a CTMS📊 Lessons learned from the selection and migration process🔗 […]
📅 Date: 20 March, 2025 ⏰ Time: 16:00 CET 🔗 Register now with just two clicks Join SOLTI and Flex Databases to discover how SOLTI transformed trial management, overcame challenges, and unlocked the full potential of digitalization. Our expert panel includes:🔹 Marietta Sarkisian – Global Director, Business Development, Flex Databases🔹 Lídice Vidal – Chief Clinical […]
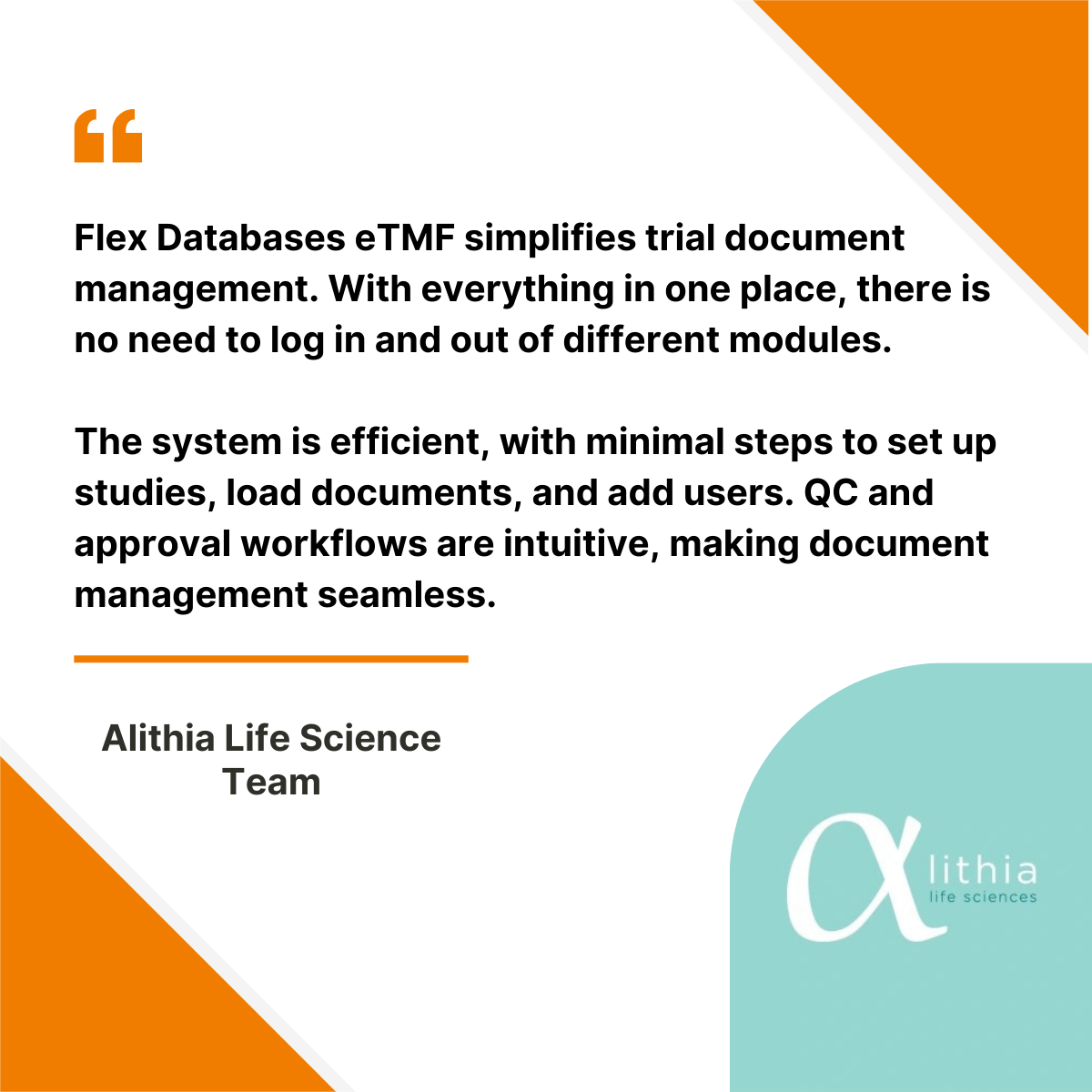
Alithia Life Sciences is a full-service Contract Research Organization dedicated to deliver high-quality clinical trial management solutions. With a strong focus on efficiency, compliance, and operational excellence, Alithia partners with pharmaceutical and biotech companies to ensure the seamless execution of clinical trials. To further enhance its document management and trial oversight, the company selected Flex […]
Join Flex Databases at key industry conferences throughout 2025! Our team will be attending major clinical research and life sciences events worldwide, ready to connect, share insights, and discuss how our platform can streamline your clinical trial management. Check out our event schedule and book a meeting with us at contact@flexdatabases.com. See you there! Date […]
A Additive Effect – The combined effect when two or more things are used together. Adherence – Following the study directions and requirements. Adverse Event – A negative change or medical occurrence that happens during a clinical trial or within a certain time period after the trial has ended. An adverse event may or may […]
At Flex Databases, we are committed to delivering cutting-edge solutions that streamline clinical trial management. Our latest updates bring powerful enhancements to the Investigators & Sites Management module and Trial Master File, helping users gain better insights, improve workflows, and ensure seamless compliance. Investigators & Sites Management: Stay Ahead with a Powerful Dashboard Our newly […]
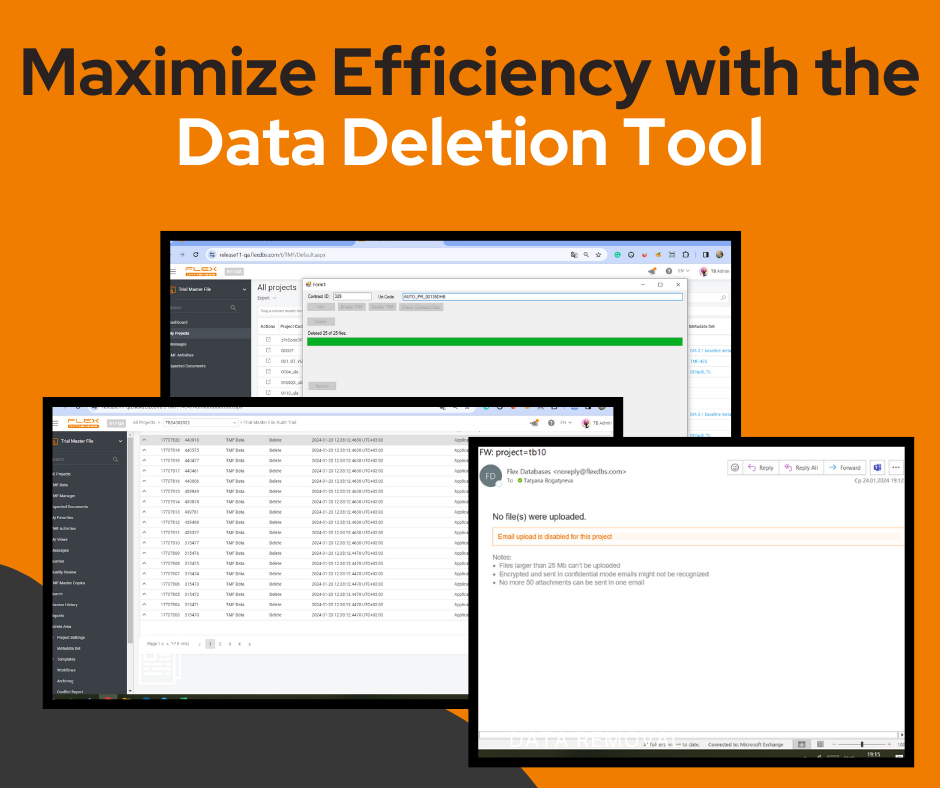
The Data Deletion Application is an advanced tool that helps organizations manage storage, meet regulations, and improve data security. It allows secure removal of entire studies from Trial Master File storage, giving businesses full control over sensitive information. Key Benefits Seamless & Secure Data Management Comprehensive Data Removal The application permanently deletes: Complete Transparency & […]