Blog Flex Databases
A Comparative Look at Europe vs. US Markets In clinical operations, time is money – but how much money can technology really save you? We analyzed the return on investment (ROI) for implementing electronic Trial Master File (eTMF) and Clinical Trial Management Systems (CTMS) based on real-world benchmarks. By modeling savings from reduced manual effort, […]
📍Remote 💼Full-Time Applications for this position are now closed and no longer being reviewed. We are driven by our high mission to make an impact in the Life Sciences industry. We provide flexible e-Clinical software systems for Clinical Research Organizations (CROs) and pharmaceutical companies. We work globally with clients all over the world, with our […]
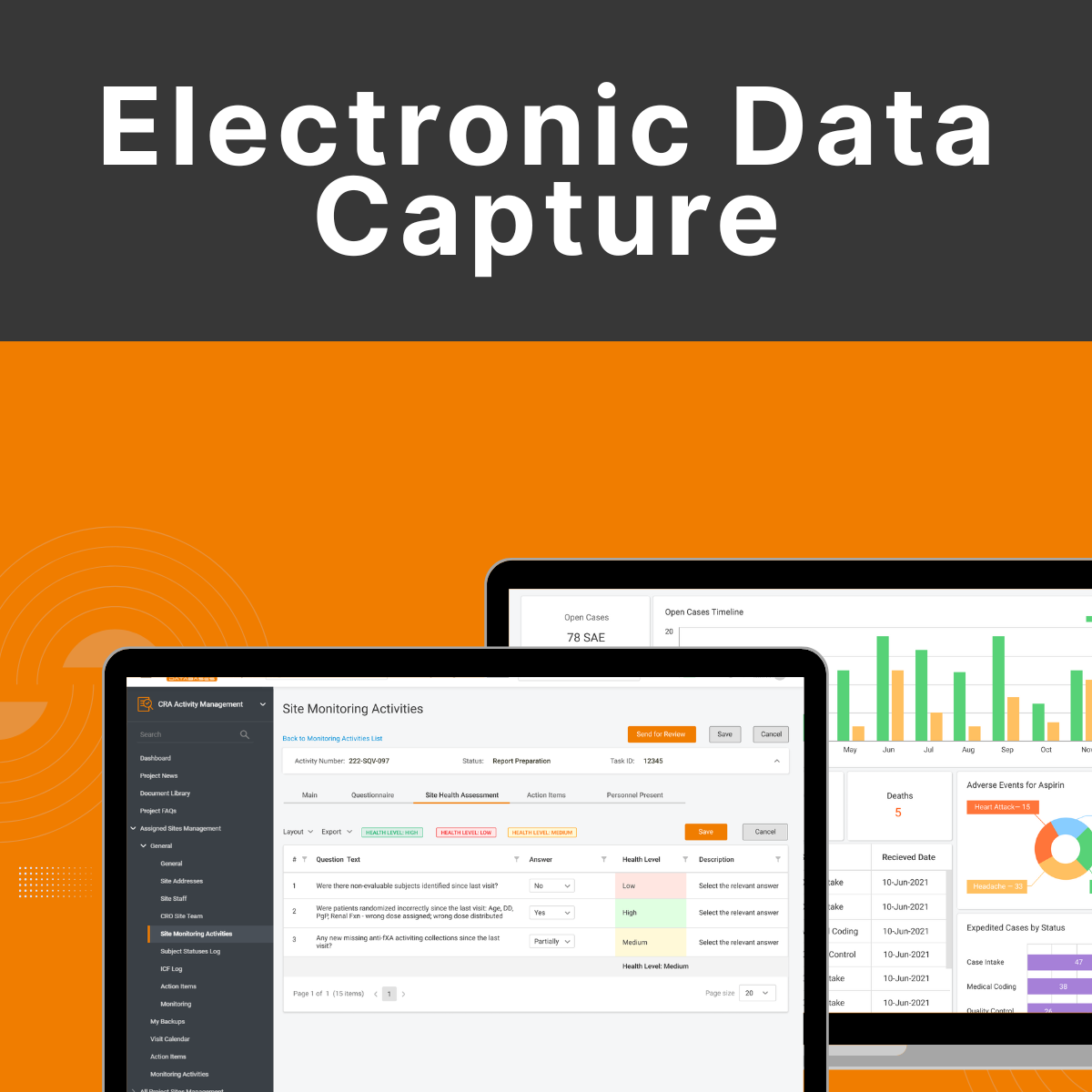
What is Electronic Data Capture? Electronic Data Capture (EDC) is an electronic system applied to clinical trials for capturing, managing, and storing patient data. Instead of paper-based forms, investigators enter clinical trial information directly into a secure, web-based site. EDC systems validate data integrity, reduce errors, and automate the data collection process – accelerating clinical […]
At Flex Databases, we deliver a SaaS solution purpose-built for clinical trials – offering global accessibility with full transparency and control over where your data resides. Not a Public Cloud. A Dedicated SaaS Platform You Can Trust. Flex Databases is an EU-based company operating globally and trusted by life sciences organizations for secure, validated SaaS […]
What is CTMS A CTMS – Clinical Trial Management System is a software solution that facilitates more streamlined management and monitoring of clinical trials. Clinical operations teams like project managers, CRAs, and study coordinators in pharmaceutical firms, CROs, and biotech firms use the tool to oversee everything from site selection and subject recruitment to monitoring […]
Dana Leff Niedzielska, MBA, SVP of Global Clinical Operations at Everest Clinical Research, shares her experience using Flex Databases’ CTMS and eTMF. In this interview, Dana highlights how Flex Databases supports Everest CRO’s clinical operations. Learn why Everest chose Flex Databases as their trusted technology partner and why they continue to rely on and trust […]
Pre-Validation for CRA Reports The Monitoring Activity report tab now features a validation preview. Users can check for missing fields and review validation messages before generating the final report – enabling more accurate submissions and reducing the need for rework. Bulk Imports for Milestones and Assumptions You can now bulk import project milestones and assumptions […]
The countdown is on – ICH GCP E6(R3) is about to go live in the EU, and everyone’s getting prepared! As a service provider (you caught that new R3 definition during training, right?), Flex Databases is here to support you through the transition. Let’s highlight a few areas that are especially important for our clients: […]
Many clinical trial teams still rely on a legacy TMF – whether that means physical binders or outdated digital folders lacking structure and traceability. These systems often fall short in today’s increasingly regulated, fast-paced environment, making oversight and compliance more difficult. That’s why more sponsors and CROs are transitioning to a modern eTMF. A modern […]
📍Remote 💼Full-Time Applications for this position are now closed and no longer being reviewed. Flex Databases is driven by our high mission to make an impact in the Life Sciences industry. We provide flexible e-Clinical software systems for Clinical Research Organizations (CROs) and pharmaceutical companies. We work globally with clients all over the world, with […]
Inside the Toolbox: eTMF, CTMS, PV, and EDC Running clinical trials today involves more than collecting data or monitoring patient visits. It’s about keeping everything connected, compliant, and on schedule while managing growing complexity. That’s where unified eClinical platforms come in, and at the core of these systems are several essential modules: eTMF, CTMS, PV, […]
Applications for this position are now closed and no longer being reviewed. We are driven by our high mission to make an impact in the Life Sciences industry. We provide flexible e-Clinical software systems for Clinical Research Organizations (CROs) and pharmaceutical companies. We work globally with clients all over the world, with our own offices […]