Blog Flex Databases
Our Head of Business Development and Marketing, Marietta Sarkisian, sat down with Ajay Sadhwani, Senior Vice President at Harbor Clinical, to talk about their experience with Flex Databases Pharmacovigilance module. In this conversation, Ajay shares how smooth implementation, responsive customer support, and the flexibility of our PV system helped Harbor Clinical streamline operations and enhance […]
Flex Databases combines deep clinical expertise with advanced technology to deliver a smarter approach to trial oversight. Our Risk-Based Monitoring (RBM) framework helps sponsors and CROs focus on what matters most, reduce effort and cost, and make faster, data-driven decisions – supported by Flex Databases’ EDC, central/remote monitoring, and AI analytics. Why Risk-Based Monitoring? How […]
📑 Tracking regulatory submissions shouldn’t require endless spreadsheets.But for many PV teams, managing ICSR reporting across agencies means manual tracking, external task lists, and risk of missed deadlines. With Flex Databases’ improved Pharmacovigilance Submissions Tracker, everything is now in one place: ✔ Define flexible submission rules by drug, project, and conditions✔ Configure statuses to match […]
Managing monitoring activities often means juggling multiple people, documents, and communication channels. Without the right tools, discussions about data discrepancies get lost in emails or chats, review cycles are disrupted, and KPIs are affected by unnecessary report declines. To solve these pains, CRAAM introduces a dedicated Query feature – designed to make handling data issues […]
Cross-site budget setup can turn into hours of manual per-subject cost entry, slowing teams down and creating error risks. With Flex Databases’ Subject Tracking & Invoicing, you can now import Per Subject Costs directly from Excel using the Data Import Wizard. ✔ Speed up site budget setup✔ Reduce manual entry and errors✔ Import across projects […]
📂 Last-minute TMF quality reviews cause stress, missed documents, and higher audit risks.With Flex Databases, you avoid the scramble – reviews are structured, transparent, and always under your control. With Flex Databases, you get:✅ Control over the process✅ Full transparency and traceability✅ Less stress before audits✅ Clear outcomes in one report 🎥 See how it […]
The FDA has just released its final guidance on Computer Software Assurance (CSA) for Production and Quality System Software. This marks a shift from traditional, document-heavy Computer System Validation (CSV) to a risk-based, critical thinking–driven approach. What does this mean? While this guidance is aimed at medical device production and quality systems, the principles perfectly […]
Ever uploaded a document to your Trial Master File only to realize it was already there?You’re not alone! Duplicates waste time, clutter your TMF, and create compliance risks. That’s why we built duplicate detection into Flex Databases eTMF. ✅ Real-time scanning as soon as you upload✅ Checks both file name and document content✅ Skip, replace, […]
Ever uploaded a document to your Trial Master File only to realize it was already there?You’re not alone! Duplicates waste time, clutter your TMF, and create compliance risks. That’s why we built duplicate detection into Flex Databases eTMF. ✅ Real-time scanning as soon as you upload✅ Checks both file name and document content✅ Skip, replace, […]
Applications for this position are now closed and no longer being reviewed. We are driven by our high mission to make an impact in the Life Sciences industry. We provide flexible e-Clinical software systems for Clinical Research Organizations (CROs) and pharmaceutical companies. We work globally with clients all over the world, with our own offices […]
When preparing a tender for a Clinical Trial Management System (CTMS) and an electronic Trial Master File (eTMF), CROs must choose solutions that meet sponsor expectations and improve operational efficiency while keeping studies inspection-ready. The right choice affects study delivery, compliance, and client satisfaction. Below are the key factors CROs should consider. Regulatory Compliance and […]
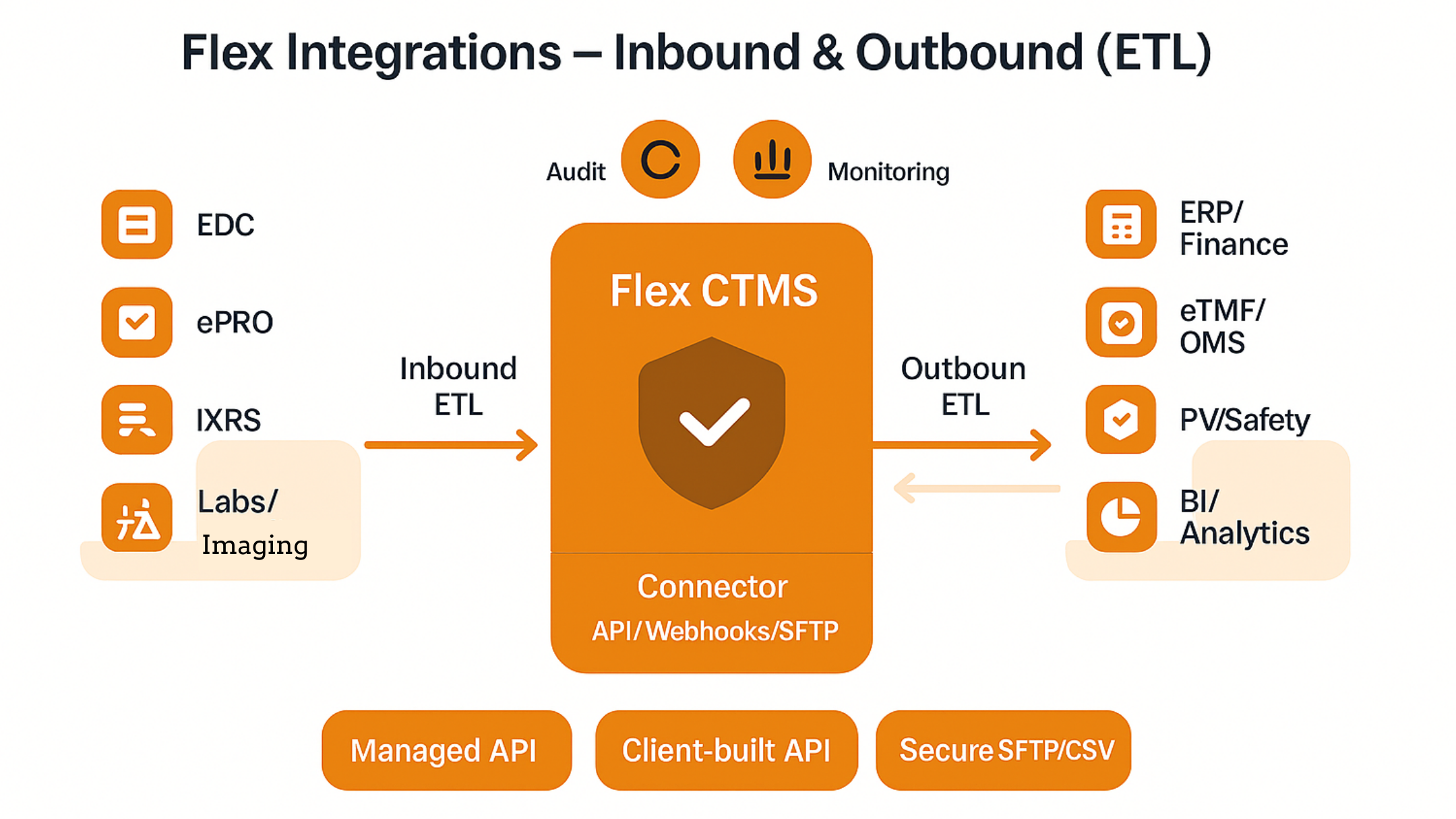
How Flex Databases connects your study ecosystem – reliably, securely, and at scale. Why integrations matter to sponsors and CROs Integration means that data entered once in a system of record is propagated automatically wherever it is needed. For clinical operations teams this translates into: Core integration patterns we see most often 1) EDC → […]