Blog Flex Databases
Palobiofarma is a leading clinical-stage biotech company dedicated to developing innovative medicines. Our focus on adenosine signaling drives our commitment to scientific excellence, with six drug candidates currently in different phases of development. Among them, PBF-999 for Prader-Willi Syndrome stands out as a key project. Our mission is to advance patient care by leveraging cutting-edge […]
At Flex Databases, we continuously improve our Trial Master File system to enhance compliance, efficiency, and user experience. Our latest update introduces an advanced Duplicates Search Feature, streamlining document management and ensuring data integrity. Introducing the Duplicates Search Feature The Duplicates Search Feature helps users identify and manage duplicate files within the TMF. It prevents […]
To simplify this process of documents efficiently management across multiple clinical projects Flex Databases introduces the Linked File feature, a powerful solution that centralizes document storage, streamlines updates, and enhances compliance. Key Features Flex Databases’ Linked Files feature centralizes key documents, connecting them to multiple projects. This eliminates the problems of managing document copies. It […]
We are thrilled to announce that Flex Databases is a proud sponsor of EUCROF 2025, taking place from February 2-4, 2025! Our team members, Tamara Prekodravac and Evgenia Michalchuk-Vrablik, will represent us at this prestigious event, where industry leaders come together to drive innovation and collaboration in clinical research. This year’s event will cover important […]
On January 14, 2025, the International Council for Harmonisation (ICH) took a major step forward in clinical trials management with the release of the updated E6(R3) Good Clinical Practice (GCP) guidelines. This milestone reflects the industry’s commitment to enhancing the quality, reliability, and compliance of clinical research. At Flex Databases, we’re proud to offer a […]
We are excited to announce several new features and improvements to Flex Databases’ modules. These updates aim to streamline workflows, enhance usability, and improve overall efficiency for our users. Here’s an overview of the latest developments and their benefits. Trial Master File (TMF) Duplicates Search: Files Content Comparison Path: Trial Master File → (open project) […]
Clinical trials are becoming more complex every year, and sponsors are raising the bar for what they expect from sites. They want faster processes, more accurate data, and better ways to keep patients engaged – all while staying compliant with ever-evolving regulations. As we look ahead to 2025, these demands will only grow. New technologies […]
We’re pleased to announce that Flex Databases’ IT security measures are now formally certified under ISO 27001. This certification, earned after a thorough audit, demonstrates that our systems meet internationally recognized standards for information security. ISO 27001 sets requirements for managing sensitive company and customer data securely. By achieving this milestone, we assure our clients […]
The Trial Master File (TMF) is the cornerstone of clinical trial documentation, serving as the definitive record of compliance with regulatory standards and ethical guidelines. Yet, the management of TMFs has grown increasingly complex with the rise in document volume, diversity, and stringent compliance requirements. Artificial intelligence (AI) is poised to address these challenges, introducing […]
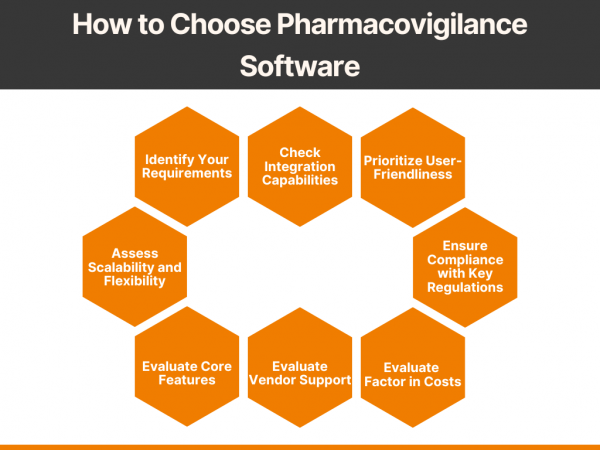
Pharmacovigilance (PV) is a critical aspect of drug safety, focusing on detecting, assessing, understanding, and preventing adverse drug reactions or other drug-related problems. As global regulatory requirements for drug safety evolve, adopting reliable pharmacovigilance software has become essential for ensuring compliance and improving operational efficiency. Selecting the right PV software can significantly impact your organization’s […]
Clinical Trial Management Systems (CTMS) and Electronic Data Capture systems are critical to the effective management of clinical trials. By integrating CTMS and EDC, organizations can streamline processes, enhance data quality, and improve overall efficiency. Here are five key tips to ensure a successful CTMS and EDC integration. Effortless Integration of Flex Databases CTMS with […]
A new round of our traditional meetings took place in October. This time, we gathered our beloved users to discuss the use of eTMF – from everyday activities to audit preparation. We reviewed how newly released features, such as duplicate search, quality review, and metadata notifications, influence TMF workflows. It appeared not everyone was using […]