Blog Flex Databases
Attending the 11th Annual Outsourcing in Clinical Trials UK and Ireland 2024? We’ll be there too! We’re thrilled to announce that Flex Databases will be participating in this prestigious event. Join us at the London Hilton on Park Lane, UK, from June 11-12. Connect with Evgenia Michalchuk-Vrablik at the event to explore our comprehensive solutions, […]
Calian expects Flex Databases to streamline their clinical trials by centralizing data, reducing manual work, and improving overall efficiency. This will lead to faster trials and better-quality information. The implementation of Flex Databases will improve Calian’s oversight capabilities and provide a single source of truth for their studies. This forward-looking approach ensures their ability to […]
Planning to attend BIO US? We are, too! We’re excited to share that Flex Databases will be participating in BIO US 2024, a premier event for the biopharma industry. Join us at the San Diego Convention Center, California, from June 3-6 📅. Connect with Matija Sokic at the event to explore our comprehensive solutions, including […]
While AI might sound futuristic, its role in document management is simply the next step in automation. Imagine a system that automatically files the documents and assigns relevant metadata. This is now possible with Flex Databases’ AI-powered solution. But before we delve deeper, let’s address a key point: AI is not a magic bullet. It’s […]
On May 21, Anna Petrovskaya, our QA Director, will be delivering a speech titled “AI-supported TMF: Validation and other Challenges” at the International QA Virtual Conference hosted by RQA. This annual event, which we eagerly attend every year, is taking place on May 21st and 22nd, 2024, under the theme ‘Back to the Future of […]
We are excited to announce Flex Databases’ participation at the 14th Annual Outsourcing in Clinical Trials Europe 2024, taking place on May 7-8 in Barcelona, Spain. We invite you to meet our team members and watch Evgenia Michalčuk-Vráblík, Global Director of Growth, giving a talk on “AI in eTMF – Auto-Classification and Metadata | Efficiency […]
What is an eTMF? An eTMF, or electronic Trial Master File, is a secure system used to manage all the essential documents and data for a clinical trial. It acts like a central library for everything related to the trial, from study protocols to investigator brochures. eTMF helps ensure everything is organized, easy to find, […]
Stallergenes Greer, a global healthcare company specializing in allergy diagnosis and treatment through allergy immunotherapy (AIT) products, has chosen Flex Databases as its eTMF vendor. The answers were provided by Virginie Lobrégat-Duriez, Director of Clinical Operations, and Dominique Jouny, Senior Clinical Project Manager. Q1. What did Stallergenes Greer have to start looking for an eTMF […]
Have questions about AI in eTMF? This is the perfect opportunity to get them answered. You can now take a picture of a document with your phone, email it to eTMF, or drag and drop a document into eTMF, and it will be automatically filed in the correct place with automatically added metadata. Embracing innovation, […]
Join us for an exclusive insightful webinar as we explore Artificial Intelligence (AI) within the Electronic Trial Master File (eTMF) system. Explore how AI can transform document management, simplify processes, and maintain compliance with regulatory standards. What to Expect? Have burning questions about AI implementation in eTMF? This webinar offers the perfect opportunity to have […]
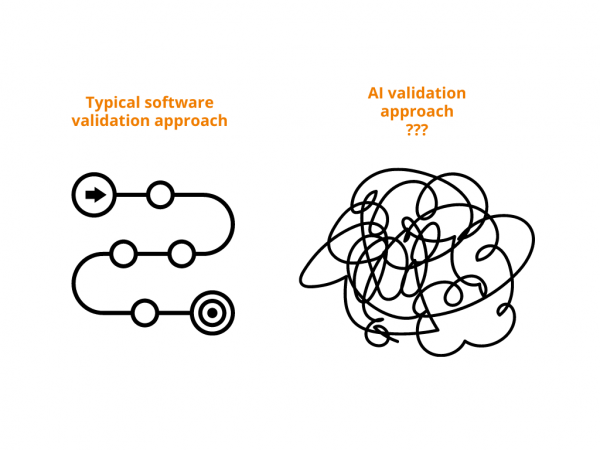
Let’s agree that in our regulated industry we take EXTRA cautious approach towards adopting new technologies. A standard validation approach of again standard computerized systems already includes This is for standard all-time favorites – EDC, eTMF, CTMS, etc. But what about if we add AI to this? With changing test results as it learns on […]
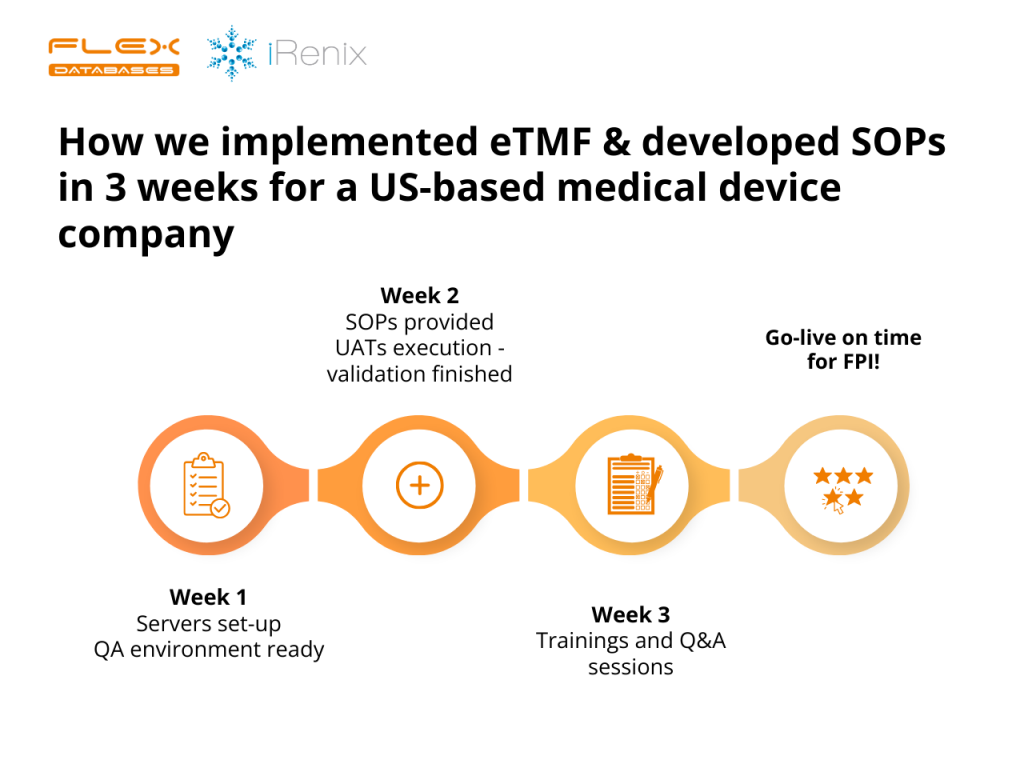
Last year, right around this time, we implemented an eTMF for a California-based Medical Device company, iRenix. Our target? A swift 3-week completion, so everything was up and running before their FPI. Now, this is quite the sprint compared to the usual 5-6 weeks! And here’s the twist – while iRenix was laser-focused on their […]