Blog Flex Databases
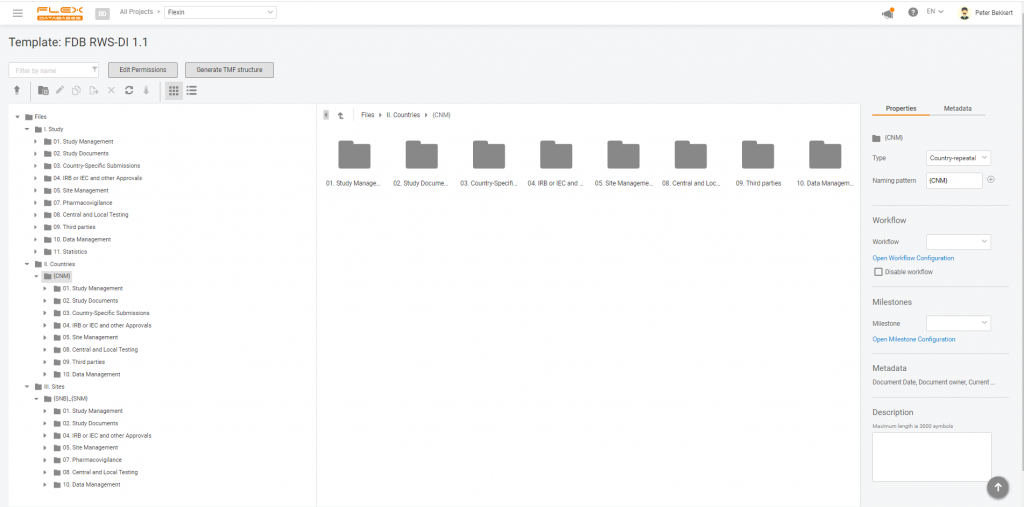
As we all know, the eTMF structure for RWE studies differs from a general TMF Reference Model. Here’s the list of main differences: To make everyone’s lives easier, we added a template for RWS to our eTMF – you can select it when you create an eTMF structure for the project from the list of […]
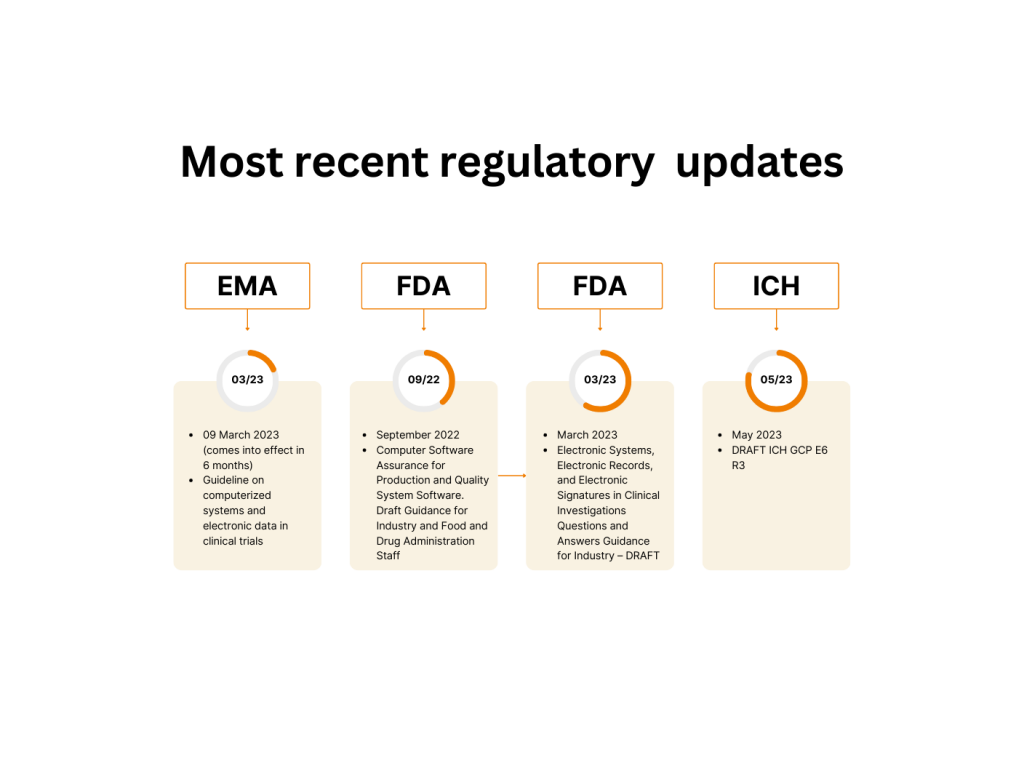
There are some eternal values in regulated companies’ lives: GCP, 21 CFR Part 11, and computerized systems validation. However, even these are subject to change. Recently the most important of them have been updated: And we can add the well-acknowledged guidance to this list: GAMP®5: 2021 Good Practice Guide: Enabling Innovation. The changed regulations do […]
On the first day of June we’ve hosted a joint webinar with our client, Confidence Pharmaceutical Research. Agenda: – Why site payments are an essential part of study management– How the site payment process works at Confidence Pharmaceutical Research/ Lifehacks of Confidence Pharmaceutical Research: how to speed up site payments in real life– More case studies: […]
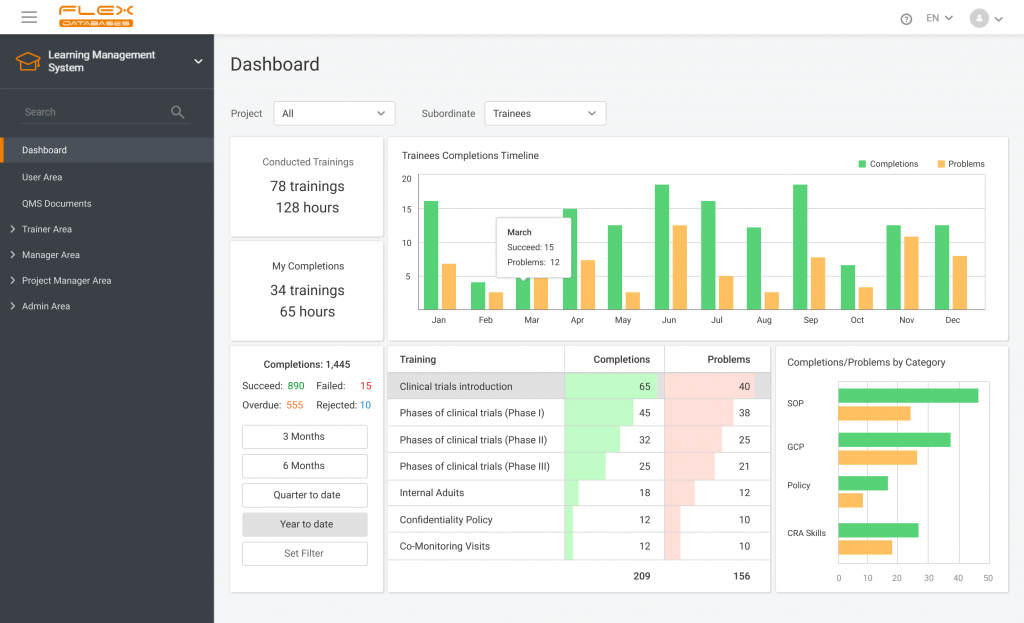
When working in a regulated industry, learning management is a huge concern since the need to follow SOPs & complete training applies to all company employees (at different levels, but still). It’s complicated enough when we think of dozens of people split between dozens of roles, but what if it comes to hundreds or thousands? […]
As spring continues to bloom, we’re excited to announce our partnership with SOLTI, Spanish academic research group that performs clinical trials of excellence in oncology. With Flex Databases CTMS & eTMF implementation, SOLTI aims to perform detailed analysis based on the information included in the system, have an operational data repository easy to consult and […]
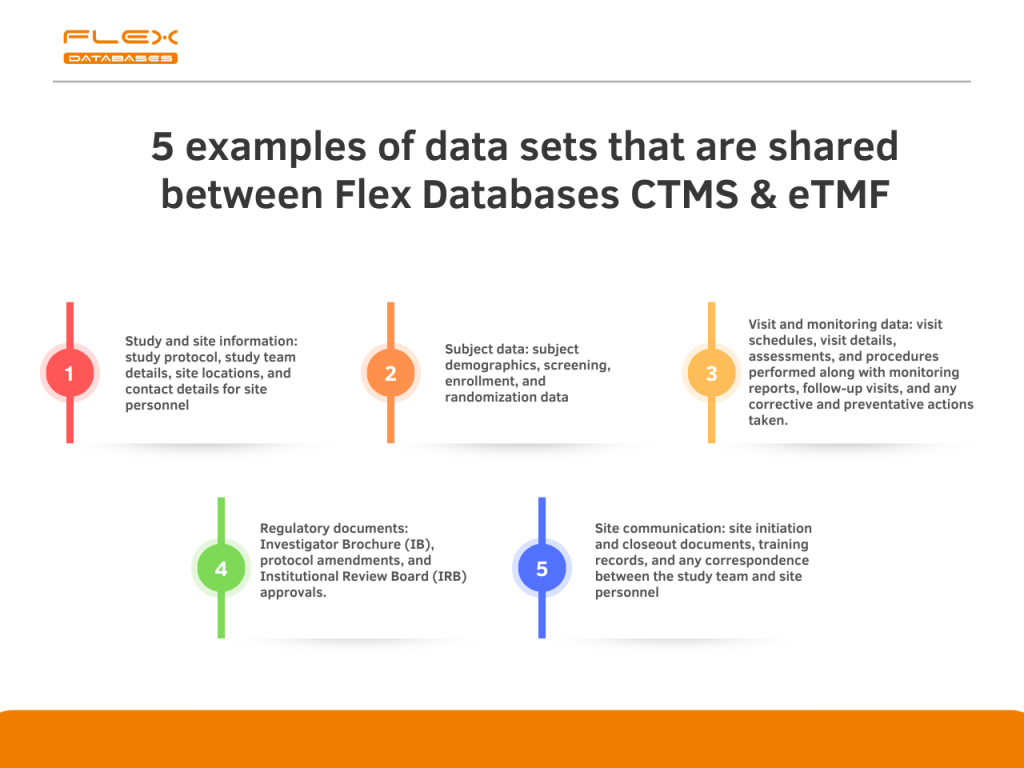
A study published in the Journal of Medical Systems found that integrating eTMF and CTMS systems resulted in a 50% reduction in the time required to identify missing documents and a 75% reduction in the time required to resolve missing document issues. Flex Databases CTMS & eTMF are interconnected to deliver you the highest possible […]
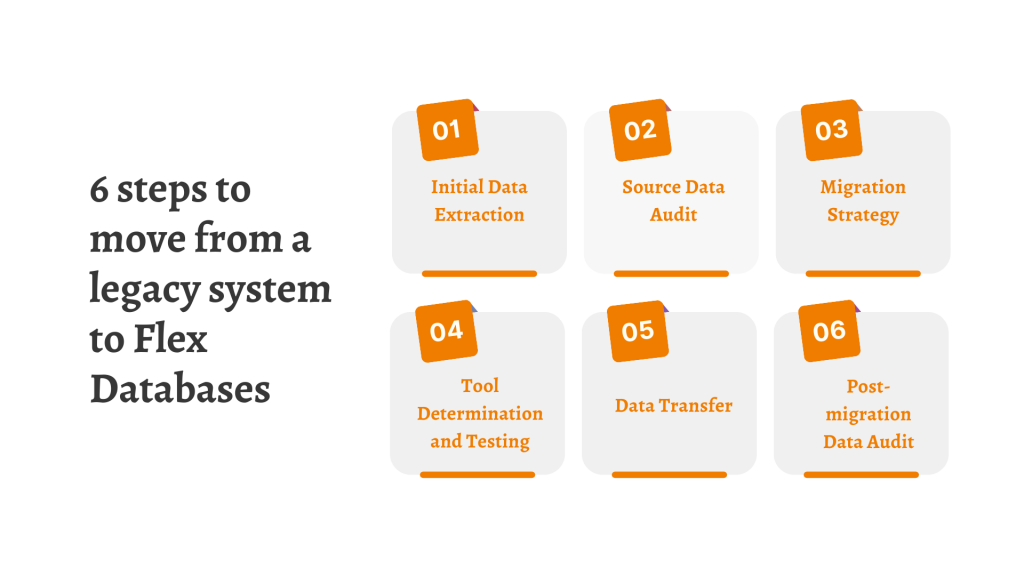
Digitalization is complicated not only the first time you go for it, but also when the moment for change comes. When we are a bit too dependant on a software provider, it may seem like there’s no way out, or data migration too much to handle – and it could be a blocker to consider […]
How long does paying to the sites while running a clinical trial take you? Average figures usually range from 4 to 6 months, even though in the contract, the payment period would typically be a month. Will you get frustrated if you are paid with a half-year delay? Yeah! Will that motivate you to work […]
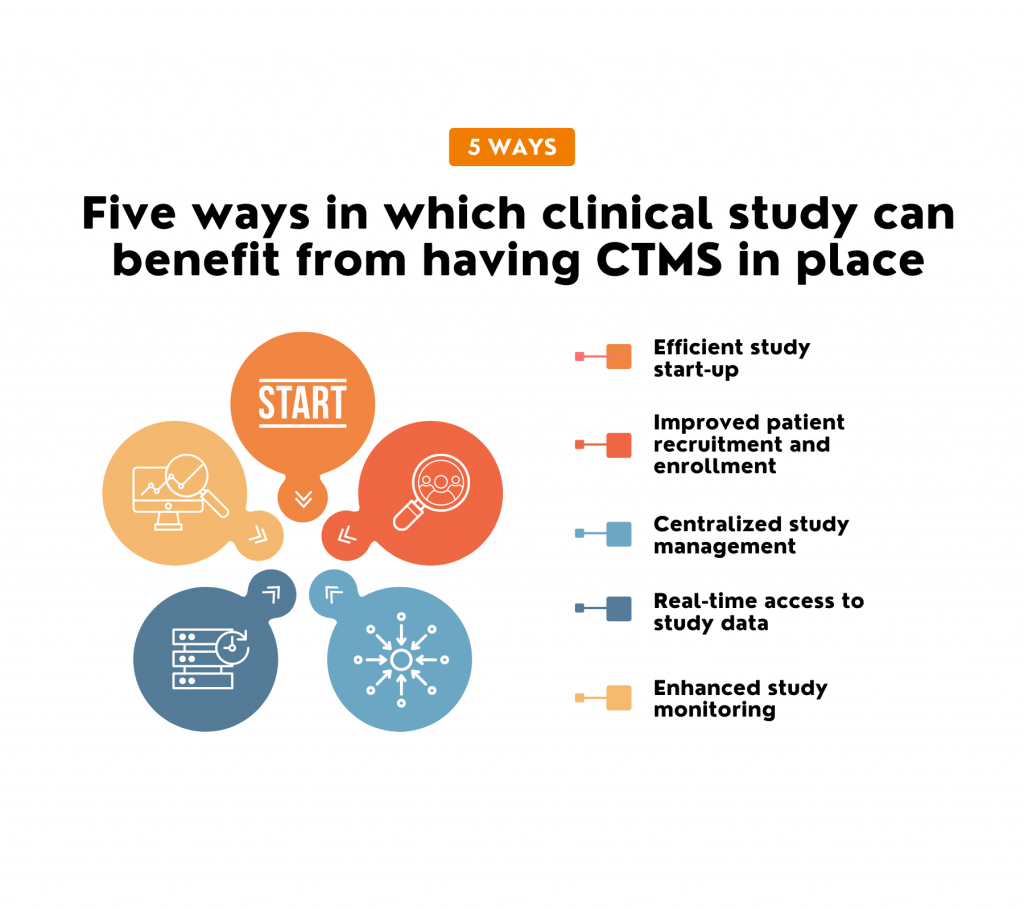
“Why did you decide to implement CTMS?” is one of the first questions our CTMS clients get during a presale. We’ve collected top-5 answers for you to check and see if something hits close to home, and it’s time to start thinking about your own CTMS implementation. Here they are: And when it comes to […]
In early April 2023 we’ve hosted a webinar about why and how you can digitalize your Quality Management System with our brilliant QA Director Anna Petrovskaya as a host. To learn more about Flex Databases QMS, please visit our product page or request a demo via form on top of the page or a request […]
Earlier this year SocraTec R&D, a CRO from Germany, selected Flex Databases as an eTMF vendor. We asked CEO André Warnke a couple of questions about the upcoming partnership: As full service CRO with all kind of clients from small/mid-sized companies to globally operating corporations, SocraTec R&D is dedicated to highest quality and user-friendly solutions […]
Early March 2023, EMA has finalised and published the updated Guideline on computerised systems and electronic data in clinical trials. Here’s how we approach compliance to Guideline requirements: EMA Guideline Flex Databases The scope of this guideline is computerised systems, (including instruments, software and ‘as a service’) used in the creation/capture of electronic clinical data […]