Blog Flex Databases
November 17th, 2022 at 17.00 CET we’ll be hosting a webinar about actionable milestones that you can use to connect & control project performance throughout the Flex Databases system, including CTMS & eTMF. Connected milestones simplify control & assessment of project performance and provide you with a full transparent picture of it. Webinar host: Mountacer Benyahya, Customer […]
This October, Metronomia Clinical Research GmbH chose Flex Databases as Project Management & Budgeting solution provider. Here’s how Metronomia’s team describes the new partnership: “We have been using Excel for many project management and budget-related activities so far. With an increasing number of projects and customers, this approach does not scale well and makes reporting […]
This September, Pharma Tech Outlook included Flex Databases in Top-10 Pharmacovigilance solutions providers in the UK & Europe. Our Business Development & Marketing Director Evgenia Mikhalchuk took part in a cover story for Pharma Tech Outlook September’22 issue, supporting our nomination: Clinical trials have always been about data. But, prior to automation, data input was […]
Send a meeting request to bd@flexdatabases.com and seh dich später!
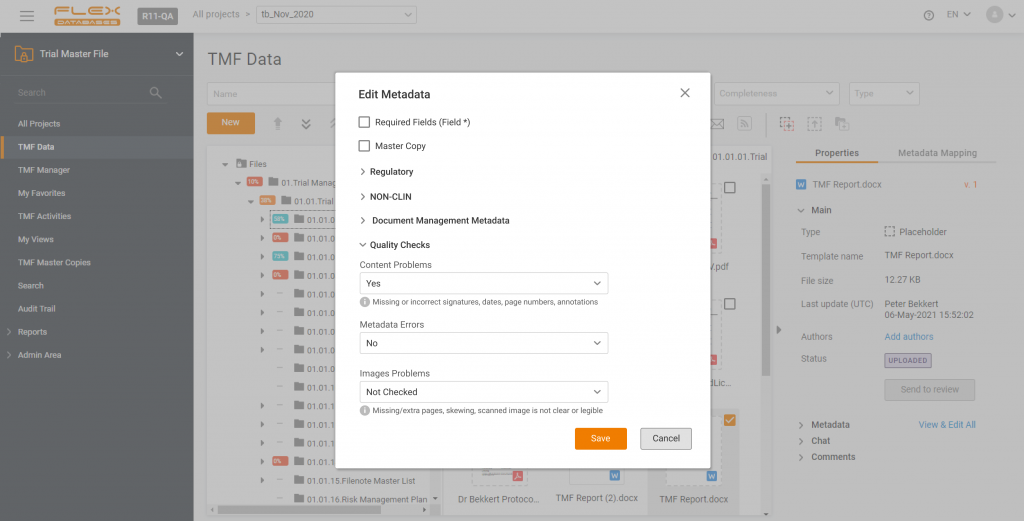
The difference between a paper Trial Master File and an electronic one is not only the storage and availability of documents. eTMF allows you to stay on top of your documentation in terms of quality – and here’s an instruction on how to set up Quality Checks in Flex Databases eTMF. In Flex Databased eTMF, […]
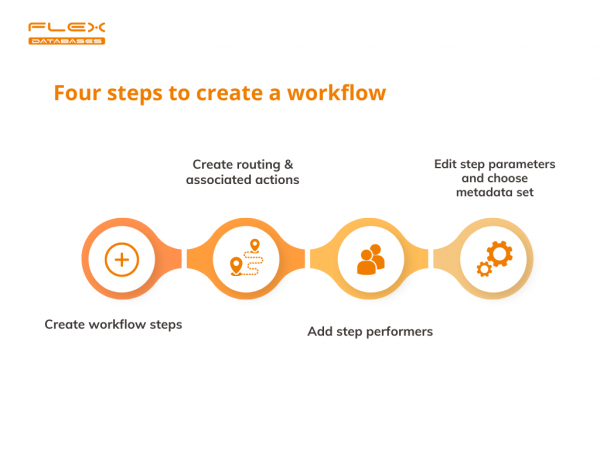
New software implementation is an imminent part of any digitalisation, but it does not come easy: out-of-the-box solutions are usually built with an average consumer in mind. It would tick the necessary and important boxes, but other than that you usually must adjust and shift your processes and procedures to it. At Flex Databases, we […]
Do you remember when each new SOP or an update of an SOP required your QA and Training Teams to send out the emails to the employees? The days when everybody had to print and sign the acknowledgment forms? And then, the poor QA and Training Teams were to file the forms manually. It seems […]
In clinical trials, any mistake can cost you a whole study. That’s why one of the essential things in the studies is processes and procedures. But what’s the first emotion you get about these words? Let me guess: disappointment, fear, irritation? It is probably connected to the fact that processes and procedures almost never work […]
This summer AXIS Clinicals chose Flex Databases as a CTMS & eTMF software provider. We’ve asked Melissa Burgard, MSW, CCRC, Director, Clinical Research and Dinkar Sindhu, Chief Executive Officer at AXIS Clinicals a few questions about the upcoming collaboration: AXIS is implementing CTMS as we see this as an excellent full-service software that will enable […]
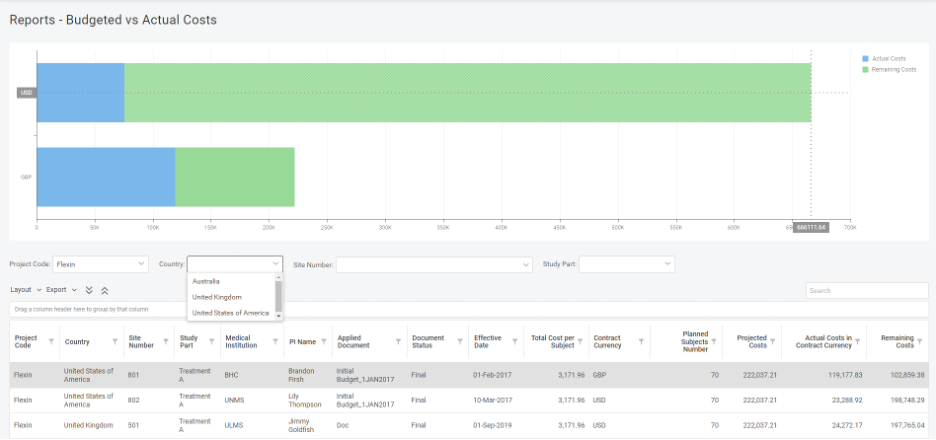
International clinical trials, in most cases, mean multiple currencies and exchange rates throughout all financial operations. Be it site or PI payments or transport and meal compensation for the patients; you will face the need to handle currency conversions in contracts and invoicing. Usually, everybody faces a lack of control and transparency when it comes […]
We celebrated an important date this year – 10 years since our first DIA Annual Meeting exhibition. Many things have changed, but the important thing has stayed the same – DIA Annual Meeting is a fantastic place to meet like-minded people, make new connections, and reconnect with old friends. We’d love to thank the event’s […]
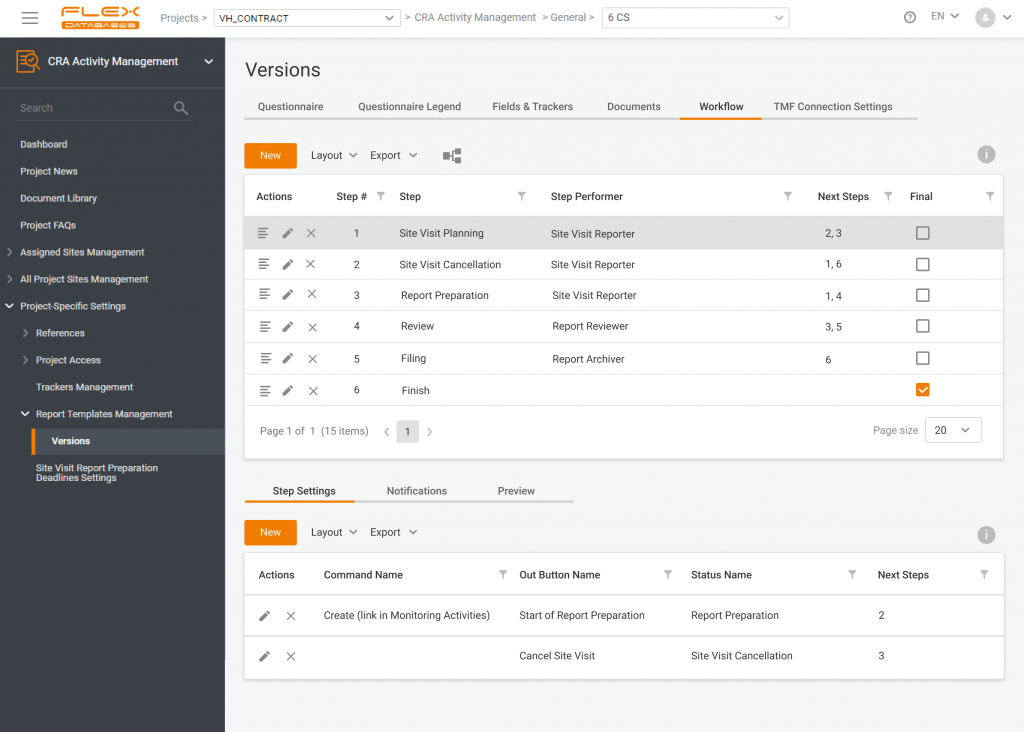
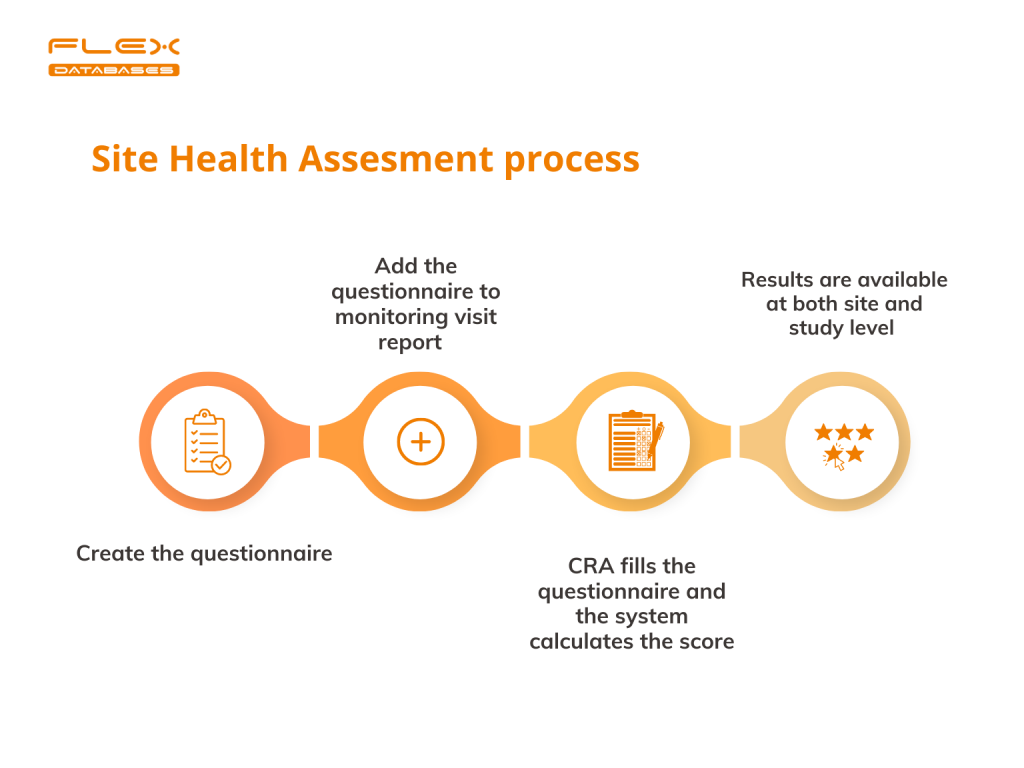
Risk-Based Monitoring (RBM) has become one of the trends in clinical trials of the last decade. Sponsors and regulatory authorities recognize RBM as an efficient approach to ensuring patient safety and data quality. We always keep track of market trends and develop features that facilitate our clients’ needs in implementing RBM techniques. What is Site […]