Blog Flex Databases
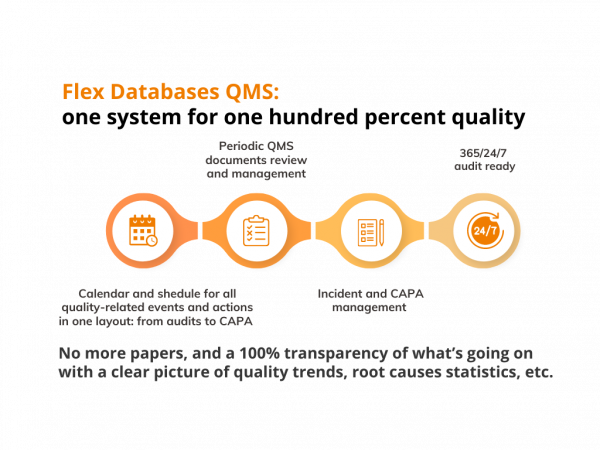
Quality Assurance is an essential part of any clinical research-related business. We built Flex Databases QMS module based on industry standards, latest trends, AND personal experience. A significant share of our employees is from QA departments of global CROs and have previous quality-related work experience. Audit calendar & reporting There’s a massive amount of internal and external […]
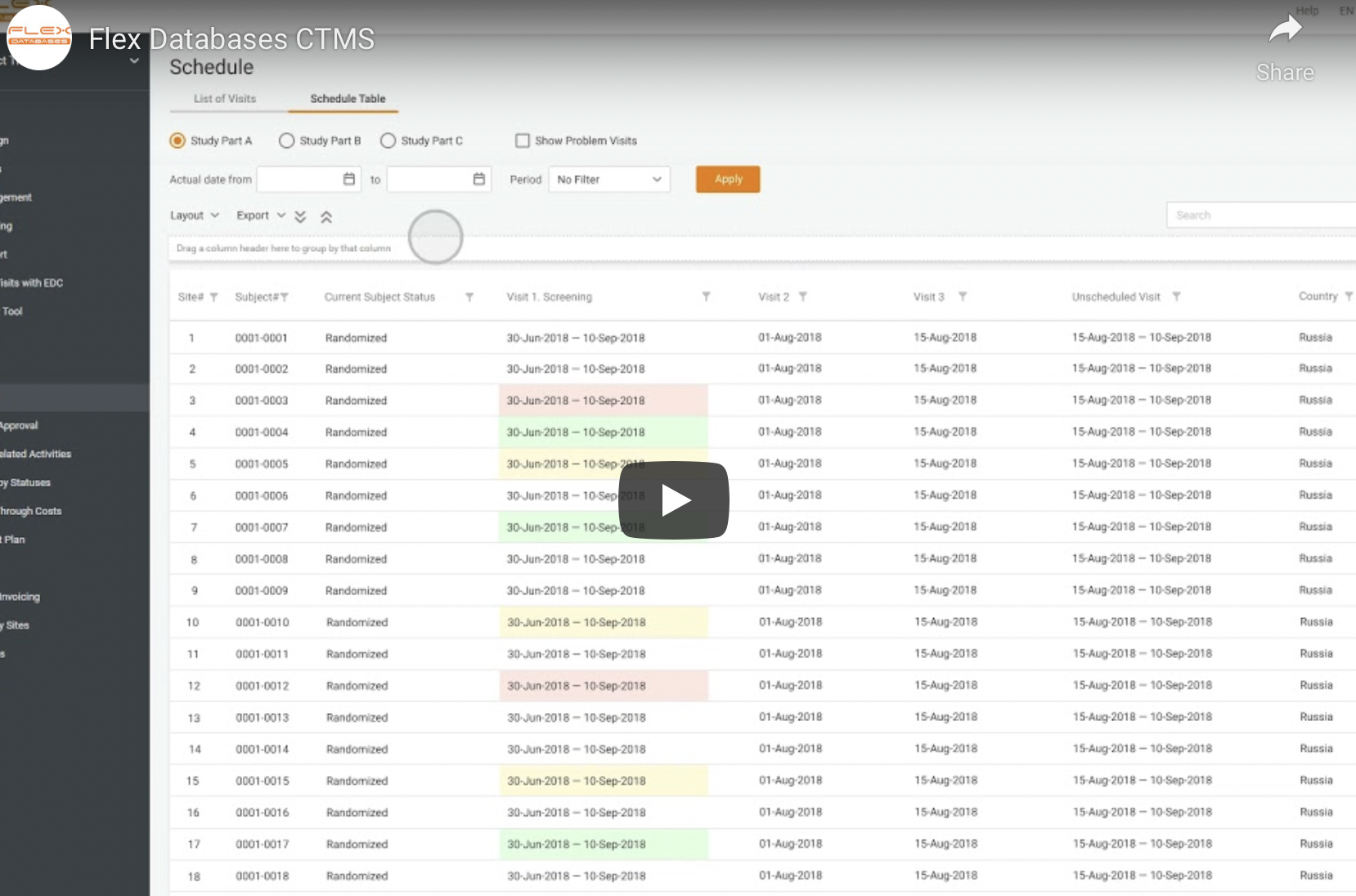
In this video, we’ll tell you about Flex Databases CTMS & eTMF implementation for FGK CRO. We’ve reviewed the case study before, you can find the text version here. Timecodes:00:19 About Flex Databases00:46 VIdeo plan01:00 About FGK CRO01:44 Project Scope02:00 Project timeline: Preparation stage02:30 QA environment02:54 Production delivery03:15 Feedback from the client03:54 Contact us
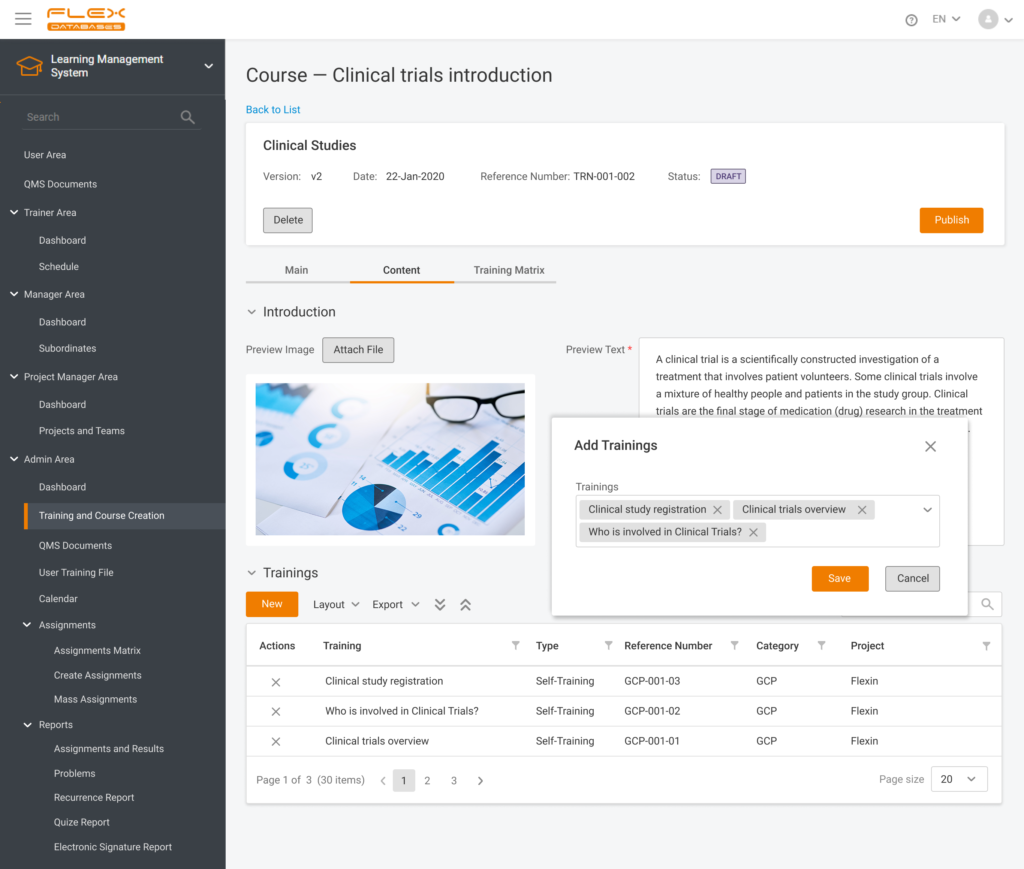
Nowadays, Learning Management System (LMS) acts as a virtual hub that helps not only with documentation, administration, reporting, tracking, delivery, and automation of training but mainly with audits and SOPs management. LMS has some crucial business benefits: There are tons of things to consider when it comes to choosing the right Learning Management System. Let’s take a […]
When you decide on the eClinical system or any other software provider, there are many questions to ask – compliance, efficiency, security, support, etc. And at the end of the day, it is also how much I pay vs. what I get for it? What about the money? How did SaaS pricing grow in proposals […]
Our QA Director, Anna Petrovskaya, will attend #RQA2021 as a speaker. Date: 03.11.2021 Time: 19:30 Theme: Vendors Input into Computer System Validation Due to travel complications during the pandemic, Anna will be a part of the Virtual Meet the Exhibitors event. You can find out more detail and book your ticket at the RQA website. The Research Quality Association […]
The development of information technology is significantly ahead of the development of regulatory and other legislation. Despite the obvious benefits of introducing new technologies in electronic systems for pharmacovigilance, it is necessary to consider the difficulties that a company will face: Validation and audits completion Personal data management Personnel training, possible changes in the organizational […]
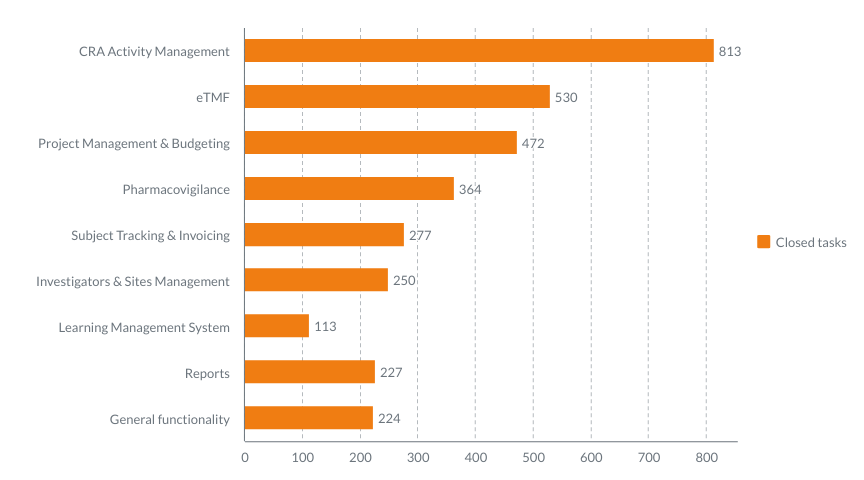
When we build a product, we base its core on the challenges our customers may face and how to solve them. We step in your shoes to see things from your point of view – what you need. Here’s the list of challenges that you have, and we solve – and how exactly we solve […]
As we briefly discussed in the previous chapter, Artificial Intelligence (AI) is used to simulate the processes of a human brain with the help of computer systems. It encompasses various technologies, including rule-following, reasoning – using rules to reach rough or specific conclusions, learning, and self-correction. Implementation areas of AI In the area of pharmacovigilance […]
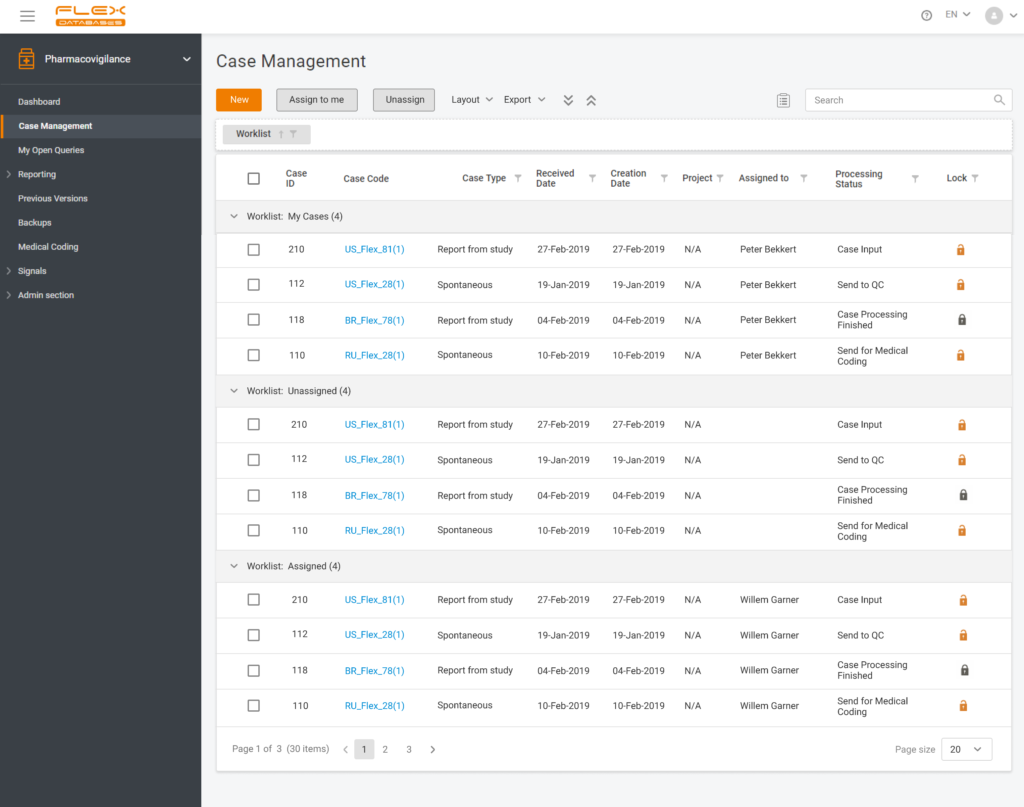
Modern pharmacovigilance, like any other process, in 2021 is impossible without technology and automation. The presence of an electronic system is a guarantee of high-quality and fast collection and processing of safety data. But even with an electronic system in place, it’s not possible to 100% avoid manual labor of collecting and entering data. But […]
So, here’s the deal: with any data point in our system, you can make a report and visualize your data. And it’s free. And it takes just a few clicks to create. How it works: Step 1: Select data sources 2. Provide permissions You can share your custom reports with anyone you like, from your […]
Clinical Trial Management System (CTMS) Manage all your CRA activities, patients’ data, sites, and invoicing in real time. Project Management & Budgeting Make the complex financial part of your project easy Electronic Trial Master File Gain complete visibility into all of your trial documentation Pharmacovigilance Ensure robust and compliant pharmacovigilance activities throughout all the trial […]
There are many factors to consider when purchasing a clinical trial management software – from regulatory requirements to the sponsor’s wishes. Many of these factors could be summarized to a need. To comply with the need, you create a checklist with major and minor features and flows to implement. But, keeping in mind that every business is […]